Engineering a hyperthermophilic archaeon for temperature-dependent product formation
- PMID: 22511351
- PMCID: PMC3345578
- DOI: 10.1128/mBio.00053-12
Engineering a hyperthermophilic archaeon for temperature-dependent product formation
Abstract
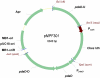
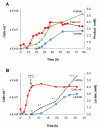
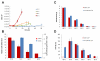
Microorganisms growing near the boiling point have enormous biotechnological potential but only recently have molecular engineering tools become available for them. We have engineered the hyperthermophilic archaeon Pyrococcus furiosus, which grows optimally at 100°C, to switch its end products of fermentation in a temperature-controlled fashion without the need for chemical inducers. The recombinant strain (LAC) expresses a gene (ldh) encoding lactate dehydrogenase from the moderately thermophilic Caldicellulosiruptor bescii (optimal growth temperature [T(opt)] of 78°C) controlled by a "cold shock" promoter that is upregulated when cells are transferred from 98°C to 72°C. At 98°C, the LAC strain fermented sugar to produce acetate and hydrogen as end products, and lactate was not detected. When the LAC strain was grown at 72°C, up to 3 mM lactate was produced instead. Expression of a gene from a moderately thermophilic bacterium in a hyperthermophilic archaeon at temperatures at which the hyperthermophile has low metabolic activity provides a new perspective to engineering microorganisms for bioproduct and biofuel formation.
Importance: Extremely thermostable enzymes from microorganisms that grow near or above the boiling point of water are already used in biotechnology. However, the use of hyperthermophilic microorganisms themselves for biotechnological applications has been limited by the lack of their genetic accessibility. Recently, a genetic system for Pyrococcus furiosus, which grows optimally near 100°C, was developed in our laboratory. In this study, we present the first heterologous protein expression system for a microorganism that grows optimally at 100°C, a first step towards the potential expression of genes involved in biomass degradation or biofuel production in hyperthermophiles. Moreover, we developed the first system for specific gene induction in P. furiosus. As the cold shock promoter for protein expression used in this study is activated at suboptimal growth temperatures of P. furiosus, it is a powerful genetic tool for protein expression with minimal interference of the host's metabolism and without the need for chemical inducers.
Figures


References
-
- Stetter KO. 2006. History of discovery of the first hyperthermophiles. Extremophiles 10:357–362 - PubMed
-
- Barnard D, Casanueva A, Tuffin M, Cowan D. 2010. Extremophiles in biofuel synthesis. Environ. Technol. 31:871–888 - PubMed
-
- Blumer-Schuette SE, Kataeva I, Westpheling J, Adams MW, Kelly RM. 2008. Extremely thermophilic microorganisms for biomass conversion: status and prospects. Curr. Opin. Biotechnol. 19:210–217 - PubMed
-
- Atomi H, Sato T, Kanai T. 2011. Application of hyperthermophiles and their enzymes. Curr. Opin. Biotechnol. 22:618–626 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
