Clinical safety, pharmacokinetics, and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension
- PMID: 22511577
- PMCID: PMC3412194
- DOI: 10.1002/ppul.22555
Clinical safety, pharmacokinetics, and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension
Abstract
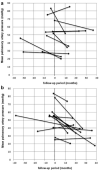
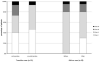
Recent trials in adult PAH revealed the efficacy of ambrisentan. However, in children with PAH, the clinical safety and pharmacokinetics of ambrisentan has not been well studied. Our aim was to investigate the clinical safety, pharmacokinetics, tolerability, and efficacy of endothelin receptor antagonist therapy with ambrisentan in children with pulmonary arterial hypertension (PAH). This retrospective cohort study provides clinical data from pediatric patients with PAH receiving ambrisentan as add-on therapy or transition from bosentan. Safety included evaluation of adverse events including aminotransferase abnormalities. The clinical impact was evaluated by improvement from baseline in clinical variables. A total of 38 pediatric patients with PAH received ambrisentan. Fifteen of 38 patients were switched from bosentan to ambrisentan. The remaining 23 children were treated with ambrisentan as an add-on therapy due to disease progression. In both transition and add-on cases, mean pulmonary artery pressure significantly improved (transition; 55 ± 18 vs. 45 ± 20 mmHg, n = 13, P = 0.04, add-on; 52 ± 17 vs. 45 ± 19 mmHg, n = 13, P = 0.03) during the follow-up. World Health Organization functional class improved in 31% of patients, but one patient required an atrial septostomy due to disease progression during the follow-up period (median, range; 20, 4-44 months). Five patients (13%) discontinued ambrisentan due to severe headache, lack of clinical efficacy, or near syncope. Ten patients (26%) had side effects associated with ambrisentan treatment, including nasal congestion, headache, and flushing. However, no patients had aminotransferase abnormalities and there were no deaths after initiation of ambrisentan during follow-up. Pharmacokinetics were evaluated in sixteen children treated with ambrisentan from 2.5 mg to 10.0 mg; the mean peak plasma concentration was 738 ± 452 ng/ml, mean time to peak plasma concentration was 3.2 ± 2.1 hours, and mean area under the curve plasma concentration was 6657 ± 4246 ng·hour/ml. In conclusion, initial experience with ambrisentan in children suggests that treatment is safe with similar pharmacokinetics to those in adults and may improve PAH in some children.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
References
-
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. - PubMed
-
- Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. - PubMed
-
- Channick RN, Sitbon O, Barst RJ, Manes A, Rubin LJ. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:62S–67S. - PubMed
-
- Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res. 2004;61:227–237. - PubMed
-
- Tsutamoto T, Wada A, Maeda Y, Adachi T, Kinoshita M. Relation between endothelin-1 spillover in the lungs and pulmonary vascular resistance in patients with chronic heart failure. J Am Coll Cardiol. 1994;23:1427–1433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

