Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity
- PMID: 22511597
- PMCID: PMC3460350
- DOI: 10.1369/0022155412446227
Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity
Abstract
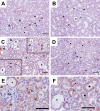
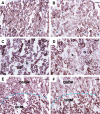
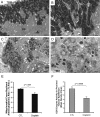
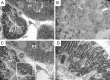
Cisplatin is a widely used antineoplastic agent. However, its major limitation is dose-dependent nephrotoxicity whose precise mechanism is poorly understood. Recent studies have suggested that mitochondrial dysfunction in tubular epithelium contributes to cisplatin-induced nephrotoxicity. Here the authors extend those findings by describing the role of an important electron transport chain enzyme, cytochrome c oxidase (COX). Immunohistochemistry for COX 1 protein demonstrated that, in response to cisplatin, expression was mostly maintained in focally damaged tubular epithelium. In contrast, COX enzyme activity in proximal tubules (by light microscopy) was decreased. Ultrastructural analysis of the cortex and outer stripe of the outer medulla showed decreased mitochondrial mass, disruption of cristae, and extensive mitochondrial swelling in proximal tubular epithelium. Functional electron microscopy showed that COX enzyme activity was decreased in the remaining mitochondria in the proximal tubules but maintained in distal tubules. In summary, cisplatin-induced nephrotoxicity is associated with structural and functional damage to the mitochondria. More broadly, using functional electron microscopy to measure mitochondrial enzyme activity may generate mechanistic insights across a spectrum of renal disorders.
Conflict of interest statement
Figures
References
-
- Aggarwal SK. 1993. A histochemical approach to the mechanism of action of cisplatin and its analogues. J Histochem Cytochem. 7:1053–1073 - PubMed
-
- Anderson WA, Bara G, Seligman AM. 1975. The ultrastructural localization of cytochrome oxidase via cytochrome. J Histochem Cytochem. 1:13–20 - PubMed
-
- Bertoni-Freddari C, Fattoretti P, Casoli T, Di Stefano G, Solazzi M, Gracciotti N, Pompei P. 2001. Mapping of mitochondrial metabolic competence by cytochrome oxidase and succinic dehydrogenase cytochemistry. J Histochem Cytochem. 9:1191–1192 - PubMed
-
- Brady HR, Kone BC, Stromski ME, Zeidel ML, Giebisch G, Gullans SR. 1990. Mitochondrial injury: an early event in cisplatin toxicity to renal proximal tubules. Am J Physiol. 258(5)(Pt 2):F1181–F1187 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

