PTTG1 oncogene promotes tumor malignancy via epithelial to mesenchymal transition and expansion of cancer stem cell population
- PMID: 22511756
- PMCID: PMC3365988
- DOI: 10.1074/jbc.M111.337428
PTTG1 oncogene promotes tumor malignancy via epithelial to mesenchymal transition and expansion of cancer stem cell population
Abstract
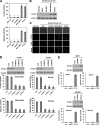
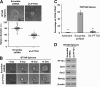
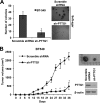
The prognosis of breast cancer patients is related to the degree of metastasis. However, the mechanisms by which epithelial tumor cells escape from the primary tumor and colonize at a distant site are not entirely understood. Here, we analyzed expression levels of pituitary tumor-transforming gene-1 (PTTG1), a relatively uncharacterized oncoprotein, in patient-derived breast cancer tissues with corresponding normal breast tissues. We found that PTTG1 is highly expressed in breast cancer patients, compared with normal tissues. Also, PTTG1 expression levels were correlated with the degree of malignancy in breast cancer cell lines; the more migratory and invasive cancer cell lines MDA-MB-231 and BT549 displayed the higher expression levels of PTTG1 than the less migratory and invasive MCF7 and SK-BR3 and normal MCF10A cell lines. By modulating PTTG1 expression levels, we found that PTTG1 enhances the migratory and invasive properties of breast cancer cells by inducing epithelial to mesenchymal transition, as evidenced by altered morphology and epithelial/mesenchymal cell marker expression patterns and up-regulation of the transcription factor Snail. Notably, down-regulation of PTTG1 also suppressed cancer stem cell population in BT549 cells by decreasing self-renewing ability and tumorigenic capacity, accompanying decreasing CD44(high) CD24(low) cells and Sox2 expression. Up-regulation of PTTG1 had the opposite effects, increasing sphere-forming ability and Sox2 expression. Importantly, PTTG1-mediated malignant tumor properties were due, at least in part, to activation of AKT, known to be a key regulator of both EMT and stemness in cancer cells. Collectively, these results suggest that PTTG1 may represent a new therapeutic target for malignant breast cancer.
Figures




References
-
- Chaffer C. L., Weinberg R. A. (2011) A perspective on cancer cell metastasis. Science 331, 1559–1564 - PubMed
-
- Liang X. (2011) EMT. New signals from the invasive front. Oral Oncol. 47, 686–687 - PubMed
-
- Christofori G. (2006) New signals from the invasive front. Nature 441, 444–450 - PubMed
-
- Karreth F., Tuveson D. A. (2004) Twist induces an epithelial-mesenchymal transition to facilitate tumor metastasis. Cancer Biol. Ther. 3, 1058–1059 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

