Vacuolar H+-ATPase (V-ATPase) promotes vacuolar membrane permeabilization and nonapoptotic death in stressed yeast
- PMID: 22511765
- PMCID: PMC3365936
- DOI: 10.1074/jbc.M112.363390
Vacuolar H+-ATPase (V-ATPase) promotes vacuolar membrane permeabilization and nonapoptotic death in stressed yeast
Abstract
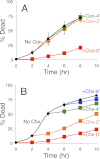
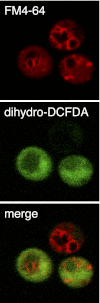
Stress in the endoplasmic reticulum caused by tunicamycin, dithiothreitol, and azole-class antifungal drugs can induce nonapoptotic cell death in yeasts that can be blocked by the action of calcineurin (Cn), a Ca(2+)-dependent serine/threonine protein phosphatase. To identify additional factors that regulate nonapoptotic cell death in yeast, a collection of gene knock-out mutants was screened for mutants exhibiting altered survival rates. The screen revealed an endocytic protein (Ede1) that can function upstream of Ca(2+)/calmodulin-dependent protein kinase 2 (Cmk2) to suppress cell death in parallel to Cn. The screen also revealed the vacuolar H(+)-ATPase (V-ATPase), which acidifies the lysosome-like vacuole. The V-ATPase performed its death-promoting functions very soon after imposition of the stress and was not required for later stages of the cell death program. Cn did not inhibit V-ATPase activities but did block vacuole membrane permeabilization (VMP), which occurred at late stages of the cell death program. All of the other nondying mutants identified in the screens blocked steps before VMP. These findings suggest that VMP is the lethal event in dying yeast cells and that fungi may employ a mechanism of cell death similar to the necrosis-like cell death of degenerating neurons.
Figures






References
-
- Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E. S., Baehrecke E. H., Blagosklonny M. V., El-Deiry W. S., Golstein P., Green D. R., Hengartner M., Knight R. A., Kumar S., Lipton S. A., Malorni W., Nuñez G., Peter M. E., Tschopp J., Yuan J., Piacentini M., Zhivotovsky B., Melino G. (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 - PMC - PubMed
-
- Golstein P., Kroemer G. (2007) Cell death by necrosis: toward a molecular definition. Trends Biochem. Sci. 32, 37–43 - PubMed
-
- Driscoll M., Gerstbrein B. (2003) Dying for a cause: invertebrate genetics takes on human neurodegeneration. Nat. Rev. Genet. 4, 181–194 - PubMed
-
- Syntichaki P., Xu K., Driscoll M., Tavernarakis N. (2002) Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 419, 939–944 - PubMed
-
- Syntichaki P., Samara C., Tavernarakis N. (2005) The vacuolar H+-ATPase mediates intracellular acidification required for neurodegeneration in C. elegans. Curr. Biol. 15, 1249–1254 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

