Mapping the environmental fitness landscape of a synthetic gene circuit
- PMID: 22511863
- PMCID: PMC3325171
- DOI: 10.1371/journal.pcbi.1002480
Mapping the environmental fitness landscape of a synthetic gene circuit
Abstract
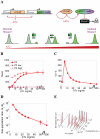
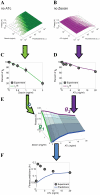
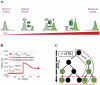
Gene expression actualizes the organismal phenotypes encoded within the genome in an environment-dependent manner. Among all encoded phenotypes, cell population growth rate (fitness) is perhaps the most important, since it determines how well-adapted a genotype is in various environments. Traditional biological measurement techniques have revealed the connection between the environment and fitness based on the gene expression mean. Yet, recently it became clear that cells with identical genomes exposed to the same environment can differ dramatically from the population average in their gene expression and division rate (individual fitness). For cell populations with bimodal gene expression, this difference is particularly pronounced, and may involve stochastic transitions between two cellular states that form distinct sub-populations. Currently it remains unclear how a cell population's growth rate and its subpopulation fractions emerge from the molecular-level kinetics of gene networks and the division rates of single cells. To address this question we developed and quantitatively characterized an inducible, bistable synthetic gene circuit controlling the expression of a bifunctional antibiotic resistance gene in Saccharomyces cerevisiae. Following fitness and fluorescence measurements in two distinct environments (inducer alone and antibiotic alone), we applied a computational approach to predict cell population fitness and subpopulation fractions in the combination of these environments based on stochastic cellular movement in gene expression space and fitness space. We found that knowing the fitness and nongenetic (cellular) memory associated with specific gene expression states were necessary for predicting the overall fitness of cell populations in combined environments. We validated these predictions experimentally and identified environmental conditions that defined a "sweet spot" of drug resistance. These findings may provide a roadmap for connecting the molecular-level kinetics of gene networks to cell population fitness in well-defined environments, and may have important implications for phenotypic variability of drug resistance in natural settings.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

