Neutralization serotyping of BK polyomavirus infection in kidney transplant recipients
- PMID: 22511874
- PMCID: PMC3325208
- DOI: 10.1371/journal.ppat.1002650
Neutralization serotyping of BK polyomavirus infection in kidney transplant recipients
Abstract
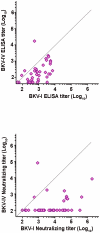
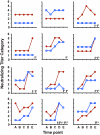
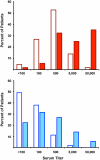
BK polyomavirus (BKV or BKPyV) associated nephropathy affects up to 10% of kidney transplant recipients (KTRs). BKV isolates are categorized into four genotypes. It is currently unclear whether the four genotypes are also serotypes. To address this issue, we developed high-throughput serological assays based on antibody-mediated neutralization of BKV genotype I and IV reporter vectors (pseudoviruses). Neutralization-based testing of sera from mice immunized with BKV-I or BKV-IV virus-like particles (VLPs) or sera from naturally infected human subjects revealed that BKV-I specific serum antibodies are poorly neutralizing against BKV-IV and vice versa. The fact that BKV-I and BKV-IV are distinct serotypes was less evident in traditional VLP-based ELISAs. BKV-I and BKV-IV neutralization assays were used to examine BKV type-specific neutralizing antibody responses in KTRs at various time points after transplantation. At study entry, sera from 5% and 49% of KTRs showed no detectable neutralizing activity for BKV-I or BKV-IV neutralization, respectively. By one year after transplantation, all KTRs were neutralization seropositive for BKV-I, and 43% of the initially BKV-IV seronegative subjects showed evidence of acute seroconversion for BKV-IV neutralization. The results suggest a model in which BKV-IV-specific seroconversion reflects a de novo BKV-IV infection in KTRs who initially lack protective antibody responses capable of neutralizing genotype IV BKVs. If this model is correct, it suggests that pre-vaccinating prospective KTRs with a multivalent VLP-based vaccine against all BKV serotypes, or administration of BKV-neutralizing antibodies, might offer protection against graft loss or dysfunction due to BKV associated nephropathy.
Conflict of interest statement
I have read the journal's policy and have the following conflict: Dr. Christopher B. Buck and Dr. Diana V. Pastrana submitted a provisional patent application entitled “Methods and Compositions for Inhibiting Polyomavirus-Associated Pathology” (Application No. 61/508,897) filed on July 18, 2011.
Figures

References
-
- Harrison JH, Merrill JP, Murray JE. Renal homotransplantation in identical twins. Surg Forum. 1956;6:432–436. - PubMed
-
- Merrill JP, Murray JE, Harrison JH, Guild WR. Successful homotransplantation of the human kidney between identical twins. J Am Med Assoc. 1956;160:277–282. - PubMed
-
- Calne RY. Cyclosporine and liver transplantation. Mt Sinai J Med. 1987;54:465–466. - PubMed
-
- Kasiske BLZM, Craig JC, Ekberg H, Garvey CA, Green MD, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. - PubMed
-
- Purighalla R, Shapiro R, McCauley J, Randhawa P. BK virus infection in a kidney allograft diagnosed by needle biopsy. Am J Kidney Dis. 1995;26:671–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

