A caprine herpesvirus 1 vaccine adjuvanted with MF59™ protects against vaginal infection and interferes with the establishment of latency in goats
- PMID: 22511971
- PMCID: PMC3325274
- DOI: 10.1371/journal.pone.0034913
A caprine herpesvirus 1 vaccine adjuvanted with MF59™ protects against vaginal infection and interferes with the establishment of latency in goats
Abstract
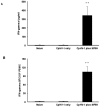
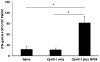
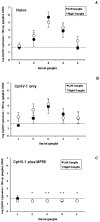
The immunogenicity and the efficacy of a beta-propiolactone-inactivated caprine herpesvirus 1 (CpHV-1) vaccine adjuvanted with MF59™ were tested in goats. Following two subcutaneous immunizations, goats developed high titers of CpHV-1-specific serum and vaginal IgG and high serum virus neutralization (VN) titers. Peripheral blood mononuclear cells (PBMC) stimulated in vitro with inactivated CpHV-1 produced high levels of soluble IFN-gamma and exhibited high frequencies of IFN-gamma producing cells while soluble IL-4 was undetectable. On the other hand, control goats receiving the inactivated CpHV-1 vaccine without adjuvant produced only low serum antibody responses. A vaginal challenge with virulent CpHV-1 was performed in all vaccinated goats and in naïve goats to assess the efficacy of the two vaccines. Vaginal disease was not detected in goats vaccinated with inactivated CpHV-1 plus MF59™ and these animals had undetectable levels of infectious challenge virus in their vaginal washes. Goats vaccinated with inactivated CpHV-1 in the absence of adjuvant exhibited a less severe disease when compared to naïve goats but shed titers of challenge virus that were similar to those of naïve goats. Detection and quantitation of latent CpHV-1 DNA in sacral ganglia in challenged goats revealed that the inactivated CpHV-1 plus MF59™ vaccine was able to significantly reduce the latent viral load when compared either to the naïve goats or to the goats vaccinated with inactivated CpHV-1 in the absence of adjuvant. Thus, a vaccine composed of inactivated CpHV-1 plus MF59™ as adjuvant was strongly immunogenic and induced effective immunity against vaginal CpHV-1 infection in goats.
Conflict of interest statement
Figures



References
-
- Saito JK, Gribble DH, Berrios PE, Knight HD, Mc Kercher DG. A new herpesvirus isolate from goats: preliminary report. Am J Vet Res. 1974;35:847–848.
-
- Van der Lugt JJ, Radles JR. Systemic herpesvirus infections in neonatal goats. J South Afr Vet Assoc. 1993;64:169–171. - PubMed
-
- Horner GW, Hunter R, Day AM. An outbreak of vulvovaginitis in goats caused by a caprine herpesvirus. NZ Vet J. 1982;30(10):150–152. - PubMed
-
- Tarigan S, Webb RF, Kirkland D. Caprine herpesvirus from balanoposthitis. Aust Vet J. 1987;64:321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

