DNA electrochemistry with tethered methylene blue
- PMID: 22512327
- PMCID: PMC3398613
- DOI: 10.1021/la300566x
DNA electrochemistry with tethered methylene blue
Abstract
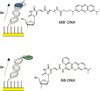
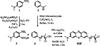
Methylene blue (MB'), covalently attached to DNA through a flexible C(12) alkyl linker, provides a sensitive redox reporter in DNA electrochemistry measurements. Tethered, intercalated MB' is reduced through DNA-mediated charge transport; the incorporation of a single base mismatch at position 3, 10, or 14 of a 17-mer causes an attenuation of the signal to 62 ± 3% of the well-matched DNA, irrespective of position in the duplex. The redox signal intensity for MB'-DNA is found to be least 3-fold larger than that of Nile blue (NB)-DNA, indicating that MB' is even more strongly coupled to the π-stack. The signal attenuation due to an intervening mismatch does, however, depend on DNA film density and the backfilling agent used to passivate the surface. These results highlight two mechanisms for reduction of MB' on the DNA-modified electrode: reduction mediated by the DNA base pair stack and direct surface reduction of MB' at the electrode. These two mechanisms are distinguished by their rates of electron transfer that differ by 20-fold. The extent of direct reduction at the surface can be controlled by assembly and buffer conditions.
Figures


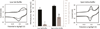



References
-
- Murphy CJ, Arkin MR, Jenkins Y, Ghatlia ND, Bossmann SH, Turro NJ, Barton JK. Long Range Photoinduced Electron Transfer through a DNA Helix. Science. 1993;262:1025–1029. - PubMed
-
- Holmlin ER, Dandliker PJ, Barton JK. Charge Transfer Through the DNA Base Stack. Angew. Chem., Int. Ed. 1997;36:2714–2730.
-
- Schuster GB, editor. Topics in Current Chemistry. Vol. 237. Berlin: Springer-Verlag; 2004. Long Range Charge Transfer in DNA II; p. 103.
-
- Wagenknecht HA, editor. Charge Transfer in DNA: From Mechanism to Application. Weiheim: Wiley-VCH Verlag GmbH & Co KGaA; 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

