Adjuvant radiotherapy for stage I endometrial cancer
- PMID: 22513918
- PMCID: PMC4164955
- DOI: 10.1002/14651858.CD003916.pub4
Adjuvant radiotherapy for stage I endometrial cancer
Abstract
Background: This is an updated version of the original Cochrane review published in Issue 2, 2007. The role of radiotherapy (both pelvic external beam radiotherapy (EBRT) and vaginal intracavity brachytherapy (VBT)) in stage I endometrial cancer following hysterectomy remains controversial.
Objectives: To assess the efficacy of adjuvant radiotherapy following surgery for stage I endometrial cancer.
Search methods: We searched The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and the Specialised Register to end-2005 for the original review, and extended the search to January 2012 for the update.
Selection criteria: We included randomised controlled trials (RCTs) that compared post-operative adjuvant radiotherapy (either EBRTor VBT, or both) versus no radiotherapy or VBT in women with stage I endometrial cancer.
Data collection and analysis: Two review authors independently assessed trials and extracted data to a specifically designed data collection form. The primary outcome was overall survival. Secondary outcomes were endometrial cancer-related deaths, locoregional recurrence and distant recurrence. Meta-analyses were performed using Cochrane Review Manager Software 5.1.
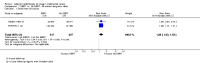
Main results: We included eight trials. Seven trials (3628 women) compared EBRT with no EBRT (or VBT), and one trial (645 women) compared VBTwith no additional treatment. We considered six of the eight trials to be of a high quality. Time-to-event data were not available for all trials and all outcomes.EBRT (with or without VBT) compared with no EBRT (or VBT alone) for stage I endometrial carcinoma significantly reduced locoregional recurrence (time-to-event data: five trials, 2965 women; Hazard Ratio (HR) 0.36, 95% Confidence Interval (CI) 0.25 to 0.52; and dichotomous data: seven trials, 3628 women; Risk Ratio (RR) 0.33, 95% CI 0.23 to 0.47). This reduced risk of locoregional recurrence did not translate into improved overall survival (time-to-event data: five trials, 2,965 women; HR 0.99, 95% CI 0.82 to1.20; and dichotomous data: seven trials, 3628 women; RR 0.98, 95% CI 0.83 to 1.15) or improved endometrial cancer-related survival (time-to-event data: five trials, 2965 women; HR 0.96, 95% CI 0.72 to 1.28; and dichotomous data: seven trials, 3628 women; RR 1.02, 95% CI 0.81 to 1.29) or improved distant recurrence rates (dichotomous data: seven trials, 3628 women; RR 1.04, 95% CI 0.80 to 1.35).EBRT did not improve survival outcomes in either the intermediate-risk or high-risk subgroups, although high-risk data were limited, and a benefit of EBRT for high-risk women could not be excluded. One trial (PORTEC-2) compared EBRT with VBT in the high-intermediate risk group and reported that VBT was effective in ensuring vaginal control with a non-significant difference in loco-regional relapse rate compared to EBRT (5.1% versus 2.1%; HR 2.08, 95% CI 0.71 to 6.09; P = 0.17). In the subgroup of low-risk patients (IA/B and grade 1/2), EBRT increased the risk of endometrial carcinoma-related deaths (including treatment-related deaths) (two trials, 517 women; RR 2.64, 95% CI 1.05 to 6.66) but there was a lack of data on overall survival. We considered the evidence for the low-risk subgroup to be of a low quality.EBRT was associated with significantly increased severe acute toxicity (two trials, 1328 patients, RR 4.68, 95% CI 1.35 to 16.16), increased severe late toxicity (six trials, 3501 women; RR 2.58, 95% CI 1.61 to 4.11) and significant reductions in quality of life scores and rectal and bladder function more than 10 years after randomisation (one trial, 351 women) compared with no EBRT.One trial of VBT versus no additional treatment in women with low-risk lesions reported a non-significant reduction in locoregional recurrence in the VBT group compared with the no additional treatment group (RR 0.39, (95% CI 0.14 to 1.09). There were no significant differences in survival outcomes in this trial.
Authors' conclusions: EBRT reduces the risk of locoregional recurrence but has no significant impact on cancer-related deaths or overall survival. It is associated with significant morbidity and a reduction in quality of life. There is no demonstrable survival advantage from adjuvant EBRT for high-risk stage I endometrial cancer, however, the meta-analyses of this subgroup were underpowered and also included high-intermediate risk women, therefore we cannot exclude a small benefit in the high-risk subgroup. EBRT may have an adverse effect on endometrial cancer survival when used to treat uncomplicated low-risk (IA/B grade 1/2) endometrial cancer. For the intermediate to high-intermediate risk group, VBT alone appears to be adequate in ensuring vaginal control compared to EBRT. Further research is needed to guide practice for lesions that are truly high risk. In addition, the definitions of risk should be standardised.
Conflict of interest statement
None known.
Figures



































Update of
-
Adjuvant radiotherapy for stage I endometrial cancer.Cochrane Database Syst Rev. 2012 Mar 14;(3):CD003916. doi: 10.1002/14651858.CD003916.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2012 Apr 18;(4):CD003916. doi: 10.1002/14651858.CD003916.pub4. PMID: 22419290 Updated.
Comment in
-
Oncology scan - gynecological cancers: new treatments, old treatments, imaging, and meta-analyses.Int J Radiat Oncol Biol Phys. 2013 Jun 1;86(2):207-10. doi: 10.1016/j.ijrobp.2013.01.026. Int J Radiat Oncol Biol Phys. 2013. PMID: 23643375 No abstract available.
References
References to studies included in this review
Aalders 1980 {published data only}
-
- Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstetrics and Gynecology 1980;56(4):419‐27. - PubMed
-
- Onsrud M, Kolstad P, Normann T. Postoperative external pelvic irradiation in carcinoma of the corpus stage I: a controlled clinical trial. Gynecologic Oncology 1976;4(2):222‐31. - PubMed
ASTEC/EN.5 {published data only}
-
- ASTEC/EN.5 Study Group: Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta‐analysis. The Lancet 2009;373(9658):137‐46. - PMC - PubMed
GOG 99 {published data only}
-
- Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. Erratum to “A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study” [Gynecol. Oncol. 92 (2004) 744–751]. Gynecologic Oncology 2004;94(1):241‐2. - PubMed
-
- Keys HM, Roberts JA, Brunetto VL, Ziano RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group Study. Gynecologic Oncology 2004;92(3):744‐51. - PubMed
-
- Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence and survival in obese women with early endometrial carcinoma: a Gynecologic Oncology Group study. Cancer 2006;107(12):2786‐91. - PubMed
PORTEC‐1 {published data only}
-
- Burger MP, Mol BW. Treatment for patients with stage‐1 endometrial carcinoma (comment on: Lancet. 2000 Apr 22;355(9213):1404‐11. Lancet 2000;356(9232):853‐4. - PubMed
-
- Creutzberg CL, Koper PCM, Lybeert MLM, Warlam Rodenhuis CC. A prospective randomized trial to assess the effect of adjuvant radiotherapy in medium risk pT1 endometrial carcinoma. International Journal of Gynecological Cancer 1995;5 (Supl.1):2 Abstract 8.
-
- Creutzberg CL, Nout RA, Lybeert ML, Wárlám‐Rodenhuis CC, Jobsen JJ, Mens JW, et al. PORTEC Study Group. Fifteen‐year radiotherapy outcomes of the randomized PORTEC‐1 trial for endometrial carcinoma. International Journal of Radiation Oncology, Biology, Physics 2011;15(81):e631‐8. - PubMed
-
- Creutzberg CL, Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage‐1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000;355(9213):1404‐11. - PubMed
-
- Creutzberg CL, Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám‐Rodenhuis CC, et al. Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecologic Oncology 2003;89(2):201‐9. - PubMed
PORTEC‐2 {published and unpublished data}
-
- Nout RA, Putter H, Jürgenliemk‐Schulz IM, Jobsen JJ, Lutgens LC, Steen‐Banasik EM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC‐2 trial. Journal of Clinical Oncology 2009;27(21):3547‐56. - PubMed
-
- Nout RA, Smit VTHBM, Putter H, Jürgenliemk‐Schulz IM, Jobsen JJ, Lutgens LCHW, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high‐intermediate risk (PORTEC‐2): an open‐label, non‐inferiority, randomised trial. The Lancet 2010;375(9717):816‐23. - PubMed
Soderini 2003 {published and unpublished data}
-
- Soderini A, Anchezar JP, Sardi JE. Role of adjuvant radiotherapy (RT) in intermediate risk (1b G2‐3‐1C) endometrioid carcinoma (EC) after extended staging surgery (ESS). Preliminary reports of a randomised trial. International Journal of Gynecological Cancer 2003;13 (Supp 1), Abstract P0147:78.
Sorbe 2009 {published and unpublished data}
-
- Sorbe B, Nordström B, Mäenpää J, Kuhelj J, Kuhelj D, Okkan S, et al. Intravaginal brachytherapy in FIGO stage I low‐risk endometrial cancer: a controlled randomized study. International Journal of Gynecological Cancer 2009;19(5):873‐8. - PubMed
Sorbe 2011 {published data only}
-
- Sorbe B, Horvath G, Andersson H, Boman K, Lundgren C, Pettersson B. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium‐risk endometrial carcinoma ‐ a prospective randomised study. International Journal of Radiation Oncology, Biology, Physics 2011 Jun 13, issue Epub ahead of print. [PUBMED: 21676554] - PubMed
References to studies excluded from this review
Algan 1996 {published data only}
-
- Algan O, Tabesh T, Hanlon A, Hogan WM, Boente M, Lanciano RM. Improved outcome in patients treated with postoperative radiation therapy for pathologic stage I/II endometrial cancer. International Journal of Radiation Oncology, Biology, Physics 1996;35(5):925‐33. - PubMed
Atzinger 2001 {published data only}
-
- Atzinger A. Is there a benefit of postoperative pelvic irradiation in patients with FIGO stage I endometrial carcinoma?. Strahlenther Onkology 2001;177(2):112‐3. - PubMed
Carey 1995 {published data only}
-
- Carey MS, O'Connell GJ, Johanson CR, Goodyear MD, Murphy KJ, Daya DM, et al. Good outcome associated with a standardized treatment protocol using selective postoperative radiation in patients with clinical stage I adenocarcinoma of the endometrium. Gynecologic Oncology 1995;57(2):138‐44. - PubMed
De Palo 1993 {published data only}
-
- Palo G, Mangioni C, Periti P, Vecchio M, Marubini E. Treatment of FIGO (1971) stage I endometrial carcinoma with intensive surgery, radiotherapy and hormonotherapy according to pathological prognostic groups. Long‐term results of a randomised multicentre study. European Journal of Cancer 1993;29A(8):1133‐40. [MEDLINE: ] - PubMed
Dickler 2010 {published data only}
DiSaia 1985 {published data only}
-
- DiSaia PJ, Creasman WT, Boronow RC, Blessing JA. Risk factors and recurrent patterns in Stage 1 endometrium cancer. American Journal of Obstetrics and Gynecology 1985;151:1009‐15. - PubMed
Eltabbakh 1997 {published data only}
-
- Eltabbakh GH, Piver MS, Hempling RE, Shin KH. Excellent long‐term survival and absence of vaginal recurrences in 332 patients with low‐risk Stage I endometrial adenocarcinoma treated with hysterectomy and vaginal brachytherapy without formal staging lymph node sampling: Report of a prospective trial. International Journal of Radiation Oncology, Biology, Physics 1997;38(2):373‐80. [MEDLINE: ] - PubMed
Garzetti 1994 {published data only}
-
- Garzetti GG, Ciavattini A, Muzzioli M, Goteri G, Fabris, N, Valensise H, et al. The relationship of clinical‐pathologic status and adjuvant treatment with natural killer cell activity in stage I and II endometrial carcinoma. Acta Obstetricia et Gynecologica Scandinavica 1994;73(8):652‐7. - PubMed
GOG 122 {published data only}
-
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole‐abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2006;24(1):36‐44. - PubMed
GOG 150 {published data only}
-
- Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin‐ifosfamide and mesna (CIM) as post‐surgical therapy in stage I‐IV carcinosarcoma (CS) of the uterus. Gynecologic Oncology 2007;107(2):177‐85. - PMC - PubMed
Haie Meder 1995 {published data only}
-
- Haie‐Meder C, Kramar A, Castaigne D, Lambin P, Bouzy J, Duvillard P, et al. Comparison of two low‐dose rates in vaginal prophylactic pre‐operative (PO) brachytherapy (BT) for stage I‐II endometrial carcinoma: results of a randomized trial. International Journal of Radiation Oncology, Biology, Physics 1995;32(Suppl1):223.
Haie Meder 2004 {published data only}
-
- Haie‐Meder C, Kramar A, Boussairi H, Bouzy J, Briot E, Castaigne D, et al. Comparison of two low dose rates in vaginal prophylactic pre‐operative brachytherapy for patients with stage I‐II endometrial carcinoma: long term results of a randomized trial. International Journal of Gynecological Cancer 2004;14:150‐1.
Hogberg 2010 {published data only}
Kang 1992 {published data only}
-
- Kang HJ, Sarin P, Lee HN, Saxena VS, Reddy S. Does treating whole abdomen improve survival in patients with clinically early stage endometrial and positive peritoneal cytology?. Gynecologic Oncology. 1992; Vol. 46:263‐4, Abstract 16.
Kucera 1990 {published data only}
-
- Kucera H, Vavra N, Weghaupt K. Benefit of external irradiation in pathologic stage I endometrial carcinoma: a prospective clinical trial of 605 patients who received postoperative vaginal irradiation and additional pelvic irradiation in the presence of unfavorable prognostic factors. Gynecologic Oncology 1990;38(1):99‐104. - PubMed
Maggi 1993 {published data only}
-
- Maggi R, Cagnazzo G, Atlante G, Marinaccio M. Risk groups and adjuvant therapy in surgical staged endometrial cancer patients. A randomized multicentre study comparing chemotherapy with radiation therapy. International Journal of Gynecological Cancer. 1999; Vol. 9 Suppl 1:85 Abstract B73.
-
- Maggi R, Cucchi L, Restelli C, Delzotti F, Frigoli A. Post surgical treatment of endometrial cancer: GICOG trial. International Journal of Gynecological Cancer. 1993; Vol. 3 Suppl 1:46 Abstract 166.
Maggi 2006 {published data only}
Marchetti 1985 {published data only}
-
- Marchetti DL, Piver MS, Tsukada Y, Reese P. 100% Prevention of vaginal recurrence in patients with stage 1, grade 1 or 2 and <50% myometrial invasion treated by TAH BSO and postoperative vaginal radium. Proceedings of American Society of Clinical Oncology 4 March 1985;4:113. [C‐439]
Meerwaldt 1990 {published data only}
-
- Meerwaldt JH, Hoekstra CJ, Putten WL, Tjokrowardojo AS, Koper PC. Endometrial adenocarcinoma, adjuvant radiotherapy tailored to prognostic factors. International Journal of Radiation Oncology Biology, Physics 1990;18:299‐304. - PubMed
Orr 1997 {published data only}
-
- Orr JW Jr, Holimon JL, Orr PF. Stage I corpus cancer: Is teletherapy necessary?. American Journal of Obstetrics and Gynaecology 1997;176(4):777‐89. - PubMed
Piver 1979 {published data only}
-
- Marchetti DL, Piver MS, Tsukada Y, Reese P. 100% prevention of vaginal recurrence in patients with stage I, grade 1 or 2 and < 50% myometrial invasion treated by TAH BSO and postoperative vaginal radium. Proceedings of ASCO 1985;4:113.
-
- Piver MS, Yazigi R, Blumenson L, Tsukada Y. A prospective trial comparing hysterectomy, hysterectomy plus vaginal radium, and uterine radium plus hysterectomy in stage I endometrial carcinoma. Obstetrics and Gynaecology 1979;54(1):85‐9. - PubMed
Poulsen 1996 {published data only}
-
- Poulsen HK, Jacobsen M, Bertelsen K, Andersen JE, Ahrons S, Bock J, et al. from The Danish Endometrial Cancer Group (DEMCA). Adjuvant radiation therapy is not necessary in the management of endometrial carcinoma stage I, low‐risk cases. International Journal of Gynecological Cancer 1996;6(1):38‐43.
Rubin 1995 {published data only}
-
- Rubin SC, Barakat RR. Treatment of high‐risk endometrial cancer should include post‐operative radiation. International Journal of Gynecological Cancer 1995;5 Suppl 1:4 Abstract 13.
Sagae 2005 {unpublished data only}
-
- Sagae S. Randomized phase III trial of whole pelvic radiotherapy vs cisplatin‐based chemotherapy in patients with intermediate risk endometrial carcinoma. Proceedings of American Society of Clinical Oncology Annual Meeting. 2005.
Sorbe 2005 {published data only}
-
- Sorbe B, Straumits, Karlsson L. Intravaginal high‐dose‐rate brachytherapy for stage I endometrial cancer: a randomized study of two dose‐per‐fraction levels. International Journal of Radiation Oncology, Biology, Physics 2005;62(5):1385‐9. - PubMed
Susumu 2008 {published data only}
-
- Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin‐based combined chemotherapy in patients with intermediate‐ and high‐risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecologic Oncology 2008;108(1):226‐33. - PubMed
Takin 2011 {published data only}
-
- Takin S, Gungor M, Orta F, Oztuna D. Does postoperative radiotherapy provide any survival advantage over observation in stage IC endometrial cancer after comprehensive surgical staging?. European Journal of Obstetrics Gynecology and Reproductive Biology 2011;154(2):200‐4. - PubMed
Touboul 2001 {published data only}
-
- Touboul E, Belkacemi Y, Buffat L, Deniaud Alexandre E, Lefranc JP, Lhuillier P, et al. Adenocarcinoma of the endometrium treated with combined irradiation and surgery: study of 437 patients. International Journal of Radiation Oncology, Biology, Physics 2001;50(1):81‐97. - PubMed
Weigensberg 1984 {published data only}
-
- Weigensberg IJ. Preoperative radiation therapy in Stage I endometrial adenocarcinoma. II. Final report of a clinical trial. Cancer 1984;53(2):242‐7. - PubMed
Additional references
Atahan 2008
-
- Atahan IL, Ozyar E, Yildiz F, Ozyigit G, Genc M, Ulger S, et al. Vaginal high dose rate brachytherapy alone in patients with intermediate to high risk stage I endometrial carcinoma after radical surgery. Internationl Journal of Gynecological Cancer 2008;18:1294‐9. - PubMed
Berrington de Gonzalez 2011
Creasman 2001
-
- Creasman WT, Odicino F, Maisonneuve P, Beller U, Benedet JL, Heintz APM, et al. Carcinoma of the corpus uteri. Journal of Epidemiology and Biostatistics 2001;6(1):45‐86. - PubMed
Creutzberg 2001
-
- Creutzberg C, Putten W, Koper P, Lybert M, Jobsen J, Warlam‐Rodenhuis C, et al. The morbidity of treatment for patients with Stage I endometrial cancer: results from a randomized trial. International Journal of Radiation Oncology, Biology , Physics 2001;51(5):1246‐55. - PubMed
Creutzberg 2003
-
- Creutzberg CL, Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám‐Rodenhuis CC, et al. Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecologic Oncology 2003;89(2):201‐9. - PubMed
Creutzberg 2004
-
- Creutzberg CL, Putten WL, Warlam‐Rodenhuis CC, Bergh AC, Winter KA, Koper PC, et al. Outcome of High‐risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. Journal of Clinical Oncology 2004;22(7):1234‐41. - PubMed
Creutzberg 2011
-
- Creutzberg CL, Nout RA, Lybeert ML, Wárlám‐Rodenhuis CC, Jobsen JJ, Mens JW, et al. PORTEC Study Group. Fifteen‐year radiotherapy outcomes of the randomized PORTEC‐1 trial for endometrial carcinoma. International Journal of Radiation Oncology, Biology, Physics 2011;15(81):3631‐8. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Higgins 2009
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011; Vol. Available from www.cochrane‐handbook.org.
Johnson 2011
Kitchener 2009
Kumar 2009
-
- Kumar S, Shah JP, Bryant CS, Awonuga AO, Imudia AN, Ruterbusch JJ, et al. Second neoplasms in survivors of endometrial cancer: impact of radiation therapy. Gynecologic Oncology 2009;113(2):233‐9. - PubMed
McCloskey 2010
-
- McCloskey SA, Tchabo NE, Malhotra HK, Odunsi K, Rodabaugh K, Singhal P, et al. Adjuvant vaginal brachytherapy alone for high risk localised endometrial cancer as defined by three major randomised trials of adjuvant pelvic radiation. Gynecologic Oncology 2010;116:404‐7. - PubMed
Naumann 2007
-
- Naumann RW, Coleman RL. The use of adjuvant radiation therapy in early endometrial cancer by members of the Society of Gynecologic Oncologists in 2005. Gynecologic Oncology 2007;105(1):7‐12. - PubMed
Nout 2011
-
- Nout RA, Poll‐Franse LV, Lybeert ML, Wárlám‐Rodenhuis CC, Jobsen JJ, Mens JW, et al. Long‐term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC‐1) trial. Journal of Clinical Oncology 2011;29(13):1692‐700. - PubMed
Panici 2008
-
- Panici PB, Basile S, Maneschi F, Lissoni AA, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early‐stage endometrial carcinoma: Randomized clinical trial. Journal of the National Cancer Institute 2008;100(23):1707‐16. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
PORTEC‐3
-
- Creutzberg CCL. Randomized Phase III Trial Comparing Concurrent Chemoradiation and Adjuvant Chemotherapy With Pelvic Radiation Alone in High Risk and Advanced Stage Endometrial Carcinoma: PORTEC‐3. Clinical Trials Register Ongoing trial; Vol. NCT00411138.
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Scholten 2005
-
- Scholten AN, Putten WL, Beerman H, Smit VT, Koper PC, Lybeert ML, et al. Postoperative radiotherapy for Stage 1 endometrial carcinoma: long‐term outcome of the randomized PORTEC trial with central pathology review. International Journal of Radiation Oncology, Biology, Physics 2005;63(3):834‐8. - PubMed
References to other published versions of this review
Kong 2007a
Kong 2007b
-
- Kong A, Simera I, Collingwood M, Williams C, Kitchener H. Adjuvant radiotherapy for stage I endometrial cancer: systematic review and meta‐analysis. Annals of Oncology 2007;18:1595‐604. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

