Regular treatment with formoterol for chronic asthma: serious adverse events
- PMID: 22513944
- PMCID: PMC4017186
- DOI: 10.1002/14651858.CD006923.pub3
Regular treatment with formoterol for chronic asthma: serious adverse events
Abstract
Background: Epidemiological evidence has suggested a link between beta(2)-agonists and increases in asthma mortality. There has been much debate about possible causal links for this association, and whether regular (daily) long-acting beta(2)-agonists are safe.
Objectives: The aim of this review is to assess the risk of fatal and non-fatal serious adverse events in trials that randomised patients with chronic asthma to regular formoterol versus placebo or regular short-acting beta(2)-agonists.
Search methods: We identified trials using the Cochrane Airways Group Specialised Register of trials. We checked websites of clinical trial registers for unpublished trial data and Food and Drug Administration (FDA) submissions in relation to formoterol. The date of the most recent search was January 2012.
Selection criteria: We included controlled, parallel design clinical trials on patients of any age and severity of asthma if they randomised patients to treatment with regular formoterol and were of at least 12 weeks' duration. Concomitant use of inhaled corticosteroids was allowed, as long as this was not part of the randomised treatment regimen.
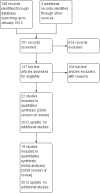
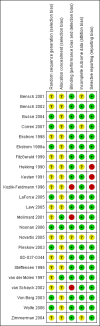
Data collection and analysis: Two authors independently selected trials for inclusion in the review. One author extracted outcome data and the second author checked them. We sought unpublished data on mortality and serious adverse events.
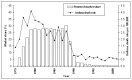
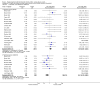
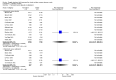
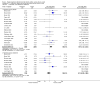
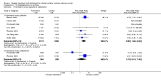
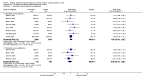
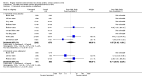
Main results: The review includes 22 studies (8032 participants) comparing regular formoterol to placebo and salbutamol. Non-fatal serious adverse event data could be obtained for all participants from published studies comparing formoterol and placebo but only 80% of those comparing formoterol with salbutamol or terbutaline.Three deaths occurred on regular formoterol and none on placebo; this difference was not statistically significant. It was not possible to assess disease-specific mortality in view of the small number of deaths. Non-fatal serious adverse events were significantly increased when regular formoterol was compared with placebo (Peto odds ratio (OR) 1.57; 95% CI 1.06 to 2.31). One extra serious adverse event occurred over 16 weeks for every 149 people treated with regular formoterol (95% CI 66 to 1407 people). The increase was larger in children than in adults, but the impact of age was not statistically significant. Data submitted to the FDA indicate that the increase in asthma-related serious adverse events remained significant in patients taking regular formoterol who were also on inhaled corticosteroids.No significant increase in fatal or non-fatal serious adverse events was found when regular formoterol was compared with regular salbutamol or terbutaline.
Authors' conclusions: In comparison with placebo, we have found an increased risk of serious adverse events with regular formoterol, and this does not appear to be abolished in patients taking inhaled corticosteroids. The effect on serious adverse events of regular formoterol in children was greater than the effect in adults, but the difference between age groups was not significant.Data on all-cause serious adverse events should be more fully reported in journal articles, and not combined with all severities of adverse events or limited to those events that are thought by the investigator to be drug-related.
Conflict of interest statement
None known.
Figures
















Update of
-
Regular treatment with formoterol for chronic asthma: serious adverse events.Cochrane Database Syst Rev. 2008 Oct 8;(4):CD006923. doi: 10.1002/14651858.CD006923.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2012 Apr 18;(4):CD006923. doi: 10.1002/14651858.CD006923.pub3. PMID: 18843738 Updated.
References
References to studies included in this review
Bensch 2001 {published data only}
-
- Bensch G, Lapidus RJ, Levine BE, Lumry W, Yegen U, Kiselev P, et al. A randomized, 12‐week, double‐blind, placebo‐controlled study comparing formoterol dry powder inhaler with albuterol metered‐dose inhaler. Annals of Allergy, Asthma, and Immunology 2001; Vol. 86, issue 1:19‐27. - PubMed
-
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003; Vol. 124, issue 1:70‐4. - PubMed
-
- Novartis. A twelve‐week, double‐blind, parallel group trial comparing the safety, tolerability and efficacy of formoterol dry powder capsules for inhalation delivered by a single‐dose inhaler versus albuterol metered‐dose inhaler (MDI) versus placebo in patients with mild to moderate asthma. FDA Application 20‐831 (Trial reference 040) Accessed 2 June 2008. [http://www.fda.gov/cder/foi/nda/2001/20831_Foradil_medr_P1.pdf ]
Bensch 2002 {published data only}
-
- Bensch G, Berger WE, Blokhin BM, Socolovsky AL, Thomson MH, Till MD, et al. One‐year efficacy and safety of inhaled formoterol dry powder in children with persistent asthma. Annals of Allergy, Asthma and Immunology 2002; Vol. 89, issue 2:180‐90. - PubMed
-
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003; Vol. 124, issue 1:70‐4. - PubMed
-
- Novartis. A twelve‐month, double‐blind, between patient placebo controlled trial comparing the safety, tolerability and efficacy of 12 mcg and 24 mcg twice daily dry powder capsules for inhalation delivered by a single‐dose inhaler (AerolizerTM) in children with asthma in need of daily treatment with inhaled bronchodilators and anti‐inflammatory treatment. FDA Application 20‐831 (Trial reference 049) Accessed 2 June 2008. [http://www.fda.gov/cder/foi/nda/2001/20831_Foradil_medr_P1.pdf ]
Busse 2004 {published data only}
-
- Busse W, Levine B, Andriano K, Lavecchia C, Yegen U. Efficacy, tolerability, and effect on asthma‐related quality of life of formoterol BID via multidose dry powder inhaler and albuterol QID via metered dose inhaler in patients with persistent asthma: a multicenter, randomized, double‐blind, double‐dummy, placebo‐controlled, parallel‐group study. Clinical Therapeutics 2004; Vol. 26, issue 10:1587‐98. - PubMed
Corren 2007 {published data only}
-
- AstraZeneca. SD‐039‐0716. A twelve‐week, randomized, double‐blind, double‐dummy, placebo‐controlled trial of SYMBICORT® pMDI (80/4.5 μg) versus its monoproducts (budesonide and formoterol) in children (≥ 6 years of age) and adults with asthma – SPRUCE 80/4.5. http://www.astrazenecaclinicaltrials.com/sites/133/imagebank/typeArticle... Accessed November 2008.
-
- Corren J, Korenblat PE, Miller CJ, O'Brien CD, Mezzanotte WS. Twelve‐week, randomized, placebo‐controlled, multicenter study of the efficacy and tolerability of budesonide and formoterol in one metered‐dose inhaler compared with budesonide alone and formoterol alone in adolescents and adults with asthma. Clinical Therapeutics 2007; Vol. 29, issue 5:823‐43. - PubMed
Ekstrom 1998 {published data only}
-
- Ekstrom T, Ringdal N, Sobradillo V, Runnerstrom E, Soliman S. Low‐dose formoterol Turbuhaler Oxis b.i.d., a 3‐month placebo‐controlled comparison with terbutaline q.i.d. Respiratory Medicine 1998; Vol. 92, issue 8:1040‐5. - PubMed
Ekstrom 1998a {published data only}
-
- Ekstrom T, Ringdal N, Tukiainen P, Runnerstrom E, Soliman S. A 3‐month comparison of formoterol with terbutaline via turbuhaler. A placebo‐controlled study. Annals of Allergy 1998; Vol. 81, issue 3:225‐30. - PubMed
FitzGerald 1999 {published data only}
-
- FitzGerald JM, Chapman KR, Della Cioppa G, Stubbing D, Fairbarn MS, Till MD, et al. Sustained bronchoprotection, bronchodilatation, and symptom control during regular formoterol use in asthma of moderate or greater severity. Journal of Allergy and Clinical Immunology 1999; Vol. 103, issue 3 I:427‐35. - PubMed
Hekking 1990 {published data only}
-
- Hekking PR, Maesen F, Greefhorst A, Prins J, Tan Y, Zweers P. Long‐term efficacy of formoterol compared to salbutamol. Lung 1990; Vol. 168, issue Suppl l:76‐82. - PubMed
Kesten 1991 {published data only}
-
- Kesten S, Chapman KR, Broder I, Cartier A, Hyland RH, Knight A, et al. A three‐month comparison of twice daily inhaled formoterol versus four times daily inhaled albuterol in the management of stable asthma. American Review of Respiratory Disease 1991; Vol. 144, issue 3 Pt 1:622‐5. - PubMed
-
- Sculpher MJ, Buxton MJ. The episode‐free day as a composite measure of effectiveness: an illustrative economic evaluation of formoterol versus salbutamol in asthma therapy [see comments]. Pharmacoeconomics 1993; Vol. 4, issue 5:345‐52. - PubMed
Kozlik‐Feldmann 1996 {published data only}
-
- Kozlik‐Feldmann R, Berg A, Berdel D, Reinhardt D. Long‐term effects of formoterol and salbutamol on bronchial hyperreactivity and beta‐adrenoceptor density on lymphocytes in children with bronchial asthma. European Journal of Medical Research 1996; Vol. 1, issue 10:465‐70. - PubMed
LaForce 2005 {published data only}
-
- LaForce C, Prenner BM, Andriano K, Lavecchia C, Yegen U. Efficacy and safety of formoterol delivered via a new multidose dry powder inhaler (Certihaler) in adolescents and adults with persistent asthma. Journal of Asthma 2005; Vol. 42, issue 2:101‐6. - PubMed
Levy 2005 {published data only}
-
- Levy R, Pinnas J, Milgrom H, Smith J, Yegen U. Safety and efficacy in children with persistent asthma treated with formoterol 10 mug BID delivered via Certihaler: a novel multidose dry‐powder inhaler. Pediatric Asthma Allergy & Immunology 2005; Vol. 18, issue 1:25‐35.
Molimard 2001 {published data only}
-
- Molimard M, Bourcereau J, Gros V, Bourdeix I, Leynadier F, Duroux P, et al. Comparison between formoterol 12 microg b.i.d. and on‐demand salbutamol in moderate persistent asthma. Respiratory Medicine 2001; Vol. 95, issue 1:64‐70. - PubMed
Noonan 2006 {published data only}
-
- Noonan M, Rosenwasser LJ, Martin P, O'Brien CD, O'Dowd L. Efficacy and safety of budesonide and formoterol in one pressurised metered‐dose inhaler in adults and adolescents with moderate to severe asthma: a randomised clinical trial. Drugs 2006; Vol. 66, issue 17:2235‐54. - PubMed
Novartis 2005 {published data only}
-
- Novartis. Pulmonary‐Allergy Drugs Advisory Committee on the safety of long‐acting beta‐agonist bronchodilators NDA 20‐831 Briefing Document. http://www.fda.gov/ohrms/dockets/AC/05/briefing/2005‐4148B1_02_01‐Novart... Accessed July 2008.
Pleskow 2003 {unpublished data only}
-
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003; Vol. 124, issue 1:70‐4. - PubMed
-
- Novartis. A twelve‐week, double‐blind, parallel group trial comparing the safety, tolerability and efficacy of formoterol dry powder capsules for inhalation delivered by a single‐dose inhaler versus albuterol metered‐dose inhaler (MDI) versus placebo in patients with mild to moderate asthma. FDA Application 20‐831 (Trial reference 041) Accessed 2 June 2008. [http://www.fda.gov/cder/foi/nda/2001/20831_Foradil_medr_P1.pdf ]
-
- Pleskow W, LaForce CF, Yegen U, Matos D, Della Cioppa G. Formoterol delivered via the dry powder Aerolizer inhaler versus albuterol MDI and placebo in mild‐to‐moderate asthma: a randomized, double‐blind, double‐dummy trial. Journal of Asthma 2003; Vol. 40, issue 5:505‐14. - PubMed
SD‐037‐0344 {unpublished data only}
-
- AstraZeneca. A 3‐month, multi‐centre, double‐blind, double‐dummy, randomised, parallel group, phase III study to investigate the efficacy and safety of formoterol HFA pMDI compared with placebo and Oxis Turbuhaler in subjects with asthma. www.astrazenecaclinicaltrials.com/Article/515841.aspx Accessed 19 June 2008.
-
- AstraZeneca. A 3‐month, multi‐centre, double‐blind, double‐dummy, randomised,parallel group, phase III study to investigate the efficacy and safety of formoterol HFA pMDI compared with placebo and Oxis® Turbuhaler® in subjects with asthma. www.astrazenecaclinicaltrials.com/sites/133/imagebank/typeArticleparam52... Accessed 19 June 2008.
Steffensen 1995 {published data only}
-
- Steffensen I, Faurschou P, Riska H, Rostrup J, Wegener T. Inhaled formoterol dry powder in the treatment of patients with reversible obstructive airway disease. A 3‐month, placebo‐controlled comparison of the efficacy and safety of formoterol and salbutamol, followed by a 12‐month trial with formoterol. Allergy 1995; Vol. 50, issue 8:657‐63. - PubMed
-
- Steffensen IE, Faurschou P. Formoterol as inhalation powder in the treatment of patients with reversible obstructive lung diseases. A 3‐month placebo‐controlled comparison of the effects of formoterol and salbutamol, followed by a 12‐month period with formoterol alone. Ugeskrift for Laeger 1996; Vol. 158, issue 49:7092‐6. - PubMed
van der Molen 1997 {published data only}
-
- Molen T. Formoterol and asthma exacerbations [5]. Chest 2004; Vol. 125, issue 4:1591. - PubMed
-
- Molen T, Postma DS, Turner MO, Jong BM, Malo JL, Chapman K, et al. Effects of the long acting beta agonist formoterol on asthma control in asthmatic patients using inhaled corticosteroids. The Netherlands and Canadian Formoterol Study Investigators. Thorax 1997; Vol. 52, issue 6:535‐9. - PMC - PubMed
-
- Molen T, Sears MR, Graaff CS, Postma DS, Meyboom‐de Jong B. Quality of life during formoterol treatment: comparison between asthma‐specific and generic questionnaires. Canadian and the Dutch Formoterol Investigators. European Respiratory Journal 1998; Vol. 12, issue 1:30‐4. - PubMed
van Schayck 2002 {published data only}
-
- Cloosterman SG, Bijl‐Hofland ID, Herwaarden CL, Akkermans RP, Elshout FJ, Folgering HT, et al. A placebo‐controlled clinical trial of regular monotherapy with short‐acting and long‐acting beta2‐agonists in allergic asthmatic patients. Chest 2001; Vol. 119, issue 5:1306‐15. - PubMed
-
- Schayck CP, Bijl‐Hofland ID, Cloosterman SG, Folgering HT, Elshout FJ, Weel C. Potential masking effect on dyspnoea perception by short‐ and long‐acting beta2‐agonists in asthma. European Respiratory Journal 2002; Vol. 19, issue 2:240‐5. - PubMed
-
- Schayck CP, Cloosterman SG, Bijl‐Hofland ID, Hoogen H, Folgering HT, Weel C. Is the increase in bronchial responsiveness or FEV1 shortly after cessation of beta2‐agonists reflecting a real deterioration of the disease in allergic asthmatic patients? A comparison between short‐acting and long‐acting beta2‐agonists. Respiratory Medicine 2002; Vol. 96, issue 3:155‐62. - PubMed
Von Berg 2003 {published data only}
-
- Berg A, Papageorgiou Saxoni F, Wille S, Carrillo T, Kattamis C, Helms PJ. Efficacy and tolerability of formoterol turbuhaler in children. International Journal of Clinical Practice 2003; Vol. 57, issue 10:852‐6. - PubMed
Wolfe 2006 {published data only}
-
- Friedman B, Sokoi W, Ziehmer B, Orevillo C, Till D. Comparable efficacy and tolerability of formoterol 12 µg or 24 µg twice daily in asthma patients [Abstract]. European Respiratory Journal 2005; Vol. 26, issue Suppl 49:Abstract No. 435.
-
- Gunkel H. FDA Clinical Review: Foradil Aerolizer. www.fda.gov/OHRMS/DOCKETS/ac/05/briefing/2005‐4148B1_03_03‐FDA‐Clinical‐... Accessed 16 June 2008.
-
- Wolfe J, LaForce C, Ostrom NK, Korenblat P, Orevillo C, Ziehmer B, et al. Formoterol 24 µg is not associated with an increase in serious asthma exacerbations in patients with persistent asthma [Abstract]. Journal of Allergy and Clinical Immunology 2005; Vol. 115, issue Suppl 2:S150.
-
- Wolfe J, LaForce C, Ziehmer B, Orevillo C, Till D. No evidence for increase in serious asthma exacerbations with formoterol 24ug twice daily [Abstract]. European Respiratory Journal 2005; Vol. 26, issue Suppl 49:Abstract No. 434.
-
- Wolfe J, Laforce C, Friedman B, Sokol W, Till D, Della Cioppa G, et al. Formoterol, 24 microg bid, and serious asthma exacerbations: similar rates compared with formoterol, 12 microg bid, with and without extra doses taken on demand, and placebo. Chest 2006; Vol. 129, issue 1:27‐38. - PubMed
Zimmerman 2004 {published data only}
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and safety of formoterol turbuhaler(R) when added to inhaled corticosteroid treatment in children with asthma. Pediatric Pulmonology 2004; Vol. 37, issue 2:122‐7. - PubMed
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and tolerability of formoterol Turbuhaler in 6‐11 year old children with asthma, not adequately controlled with inhaled corticosteroids [abstract]. European Respiratory Society Annual Congress 2002 2002:Abstract no: P2734.
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and tolerability of formoterol turbuhaler compared with placebo in children (6‐11 years) with asthma poorly controlled with inhaled corticosteroids. American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A746.
References to studies excluded from this review
Adler 2006 {published data only}
-
- Adler A, Uziel Y, Mei‐Zahav M, Horowitz I. Formoterol induces tolerance to the bronchodilating effect of salbutamol following methacholine‐provocation test in asthmatic children. Pulmonary Pharmacology and Therapeutics 2006; Vol. 19, issue 4:281‐5. - PubMed
Akpinarli 1999 {published data only}
Ankerst 2003 {published data only}
-
- Ankerst J, Persson G, Weibull E. Tolerability of a high dose of budesonide/formoterol in a single inhaler in patients with asthma. Pulmonary Pharmacology and Therapeutics 2003; Vol. 16, issue 3:147‐51. - PubMed
Arvidsson 1989 {published data only}
-
- Arvidsson P, Larsson S, Lofdahl CG, Melander B, Wahlander L, Svedmyr N. Formoterol, a new long‐acting bronchodilator for inhalation. European Respiratory Journal 1989; Vol. 2, issue 4:325‐30. - PubMed
Aziz 1998 {published data only}
-
- Aziz I, McFarlane LC, Lipworth BJ. Concomitant inhaled corticosteroid resensitises cardiac beta2‐adrenoceptors in the presence of long‐acting beta2‐agonist therapy. European Journal of Clinical Pharmacology 1998; Vol. 54, issue 5:377‐81. - PubMed
Bauer 1995 {published data only}
-
- Bauer K, Sertl K, Kaik B, Kaik G. Effect of solution and suspension type aerosol of formoterol on tremor response and airways in patients with asthma. Journal of Allergy and Clinical Immunology 1995; Vol. 96, issue 4:495‐501. - PubMed
Behling 1999 {published data only}
-
- Behling B, Matthys H. Comparison of efficacy and safety of formoterol with terbutaline in children with mild to moderate asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain 1999:P369.
Boner 1994 {published data only}
-
- Boner AL, Spezia E, Piovesan P, Chiocca E, Maiocchi G. Inhaled formoterol in the prevention of exercise‐induced bronchoconstriction in asthmatic children. American Journal of Respiratory and Critical Care Medicine 1994; Vol. 149, issue 4 Pt 1:935‐9. - PubMed
Bousquet 2005 {published data only}
-
- Bousquet J, Guenole E, Duvauchelle T, Vicaut E, Lefrancois G. A randomized, double‐blind, double‐dummy, single‐dose, crossover trial evaluating the efficacy and safety profiles of two dose levels (12 and 24 microg) of formoterol‐HFA (pMDI) vs. those of a dose level (24 microg) of formoterol‐DPI (Foradil/Aerolizer) and of placebo (pMDI or Aerolizer) in moderate to severe asthmatic patients. Respiration 2005; Vol. 72, issue Suppl 1:13‐9. - PubMed
Bousquet 2005a {published data only}
-
- Bousquet J, Huchon G, Leclerc V, Vicaut E, Lefrancois G. A randomized, double‐blind, double‐dummy, single‐dose, efficacy crossover trial comparing formoterol‐HFA (pMDI) versus formoterol‐DPI (Aerolizer) and placebo (pMDI or Aerolizer) in asthmatic patients. Respiration 2005; Vol. 72, issue Suppl 1:6‐12. - PubMed
Brambilla 2003 {published data only}
-
- Brambilla C, Gros V, Bourdeix I, Efficacy of Foradil in Asthma French Study G. Formoterol 12 microg BID administered via single‐dose dry powder inhaler in adults with asthma suboptimally controlled with salmeterol or on‐demand salbutamol: a multicenter, randomized, open‐label, parallel‐group study. Clinical Therapeutics 2003; Vol. 25, issue 7:2022‐36. - PubMed
Bronsky 2002 {published data only}
-
- Bronsky EA, Yegen U, Yeh CM, Larsen LV, Della Cioppa G. Formoterol provides long‐lasting protection against exercise‐induced bronchospasm. Annals of Allergy, Asthma and Immunology 2002; Vol. 89, issue 4:407‐12. - PubMed
Bronsky 2004 {published data only}
-
- Bronsky EA, Grossman J, Henis MJ, Gallo PP, Yegen U, Della Cioppa G, et al. Inspiratory flow rates and volumes with the Aerolizer dry powder inhaler in asthmatic children and adults. Current Medical Research & Opinion 2004; Vol. 20, issue 2:131‐7. - PubMed
Brusasco 2002 {published data only}
-
- Brusasco V, Crimi E, Gherson G, Nardelli R, Oldani V, Francucci B, et al. Actions other than smooth muscle relaxation may play a role in the protective effects of formoterol on the allergen‐induced late asthmatic reaction. Pulmonary Pharmacology & Therapeutics 2002; Vol. 15, issue 4:399‐406. - PubMed
Burgess 1998 {published data only}
-
- Burgess C, Ayson M, Rajasingham S, Crane J, Della Cioppa G, Till MD. The extrapulmonary effects of increasing doses of formoterol in patients with asthma. European Journal of Clinical Pharmacology 1998; Vol. 54, issue 2:141‐7. - PubMed
Cazzola 2002 {published data only}
-
- Cazzola M, Grella E, Matera MG, Mazzarella G, Marsico SA. Onset of action following formoterol Turbuhaler and salbutamol pMDI in reversible chronic airway obstruction. Pulmonary Pharmacology and Therapeutics 2002; Vol. 15, issue 2:97‐102. - PubMed
Ceylan 2004 {published data only}
-
- Ceylan E, Mehmet G, Sahin A. Addition of formoterol or montelukast to low‐dose budesonide: an efficacy comparison in short‐ and long‐term asthma control. Respiration 2004; Vol. 71, issue 6:594‐601. - PubMed
Chetta 1993 {published data only}
-
- Chetta A, Donno M, Maiocchi G, Pisi G, Moretti D, Olivieri D. Prolonged bronchodilating effect of formoterol versus procaterol in bronchial asthma. Annals of Allergy 1993; Vol. 70, issue 2:171‐4. - PubMed
Cheung 2006 {published data only}
-
- Cheung D, Klink HCJ, Aalbers R. Improved lung function and symptom control with formoterol on demand in asthma. European Respiratory Journal 2006; Vol. 27, issue 3:504‐10. - PubMed
Chuchalin 2002 {published data only}
-
- Chuchalin AG, Ovcharenko SI, Goriachkina LA, Sidorenko IV, Tsoi AN, Epoch Study G. The safety and efficacy of formoterol OxisR TurbuhalerR plus budesonide PulmicortR Turbuhaler in mild to moderate asthma: A comparison with budesonide Turbuhaler alone and current non‐corticosteroid therapy in Russia. International Journal of Clinical Practice 2002; Vol. 56, issue 1:15‐20. - PubMed
Chuchalin 2005 {published data only}
-
- Chuchalin A, Kasl M, Bengtsson T, Nihlen U, Rosenborg J. Formoterol used as needed in patients with intermittent or mild persistent asthma. Respiratory Medicine 2005; Vol. 99, issue 4:461‐70. - PubMed
Chuchalin 2005a {published data only}
-
- Chuchalin AG, Manjra AI, Rozinova NN, Skopkova O, Cioppa GD, Till D, et al. Formoterol delivered via a new multi‐dose dry powder inhaler (Certihaler) is as effective and well tolerated as the formoterol dry powder inhaler (Aerolizer) in children with persistent asthma. Journal of Aerosol Medicine 2005; Vol. 18, issue 1:63‐73. - PubMed
Chung 1995 {published data only}
-
- Chung KF. Eformoterol and bronchial hyperresponsiveness in mild asthma. British Journal of Clinical Practice. Supplement 1995, issue 81:14‐5. - PubMed
Dahl 2004 {published data only}
-
- Dahl R, Creemers JP, Noord J, Sips A, Della Cioppae G, Thomson M, et al. Comparable efficacy and tolerability of formoterol (Foradil) administered via a novel multi‐dose dry powder inhaler (Certihaler) or the Aerolizer dry powder inhaler in patients with persistent asthma. Respiration 2004; Vol. 71, issue 2:126‐33. - PubMed
Daugbjerg 1996 {published data only}
-
- Daugbjerg P, Nielsen KG, Skov M, Bisgaard H. Duration of action of formoterol and salbutamol dry‐powder inhalation in prevention of exercise‐induced asthma in children. Acta Paediatrica 1996; Vol. 85, issue 6:684‐7. - PubMed
Dey 2005 {published data only}
-
- Dey. Study in patients with asthma. Clinicaltrials.gov 2005.
Dietrich 2006 {published data only}
-
- Dietrich H, Nguyen TD, Buermann U, Petzold U, Munzel U, Maus J. Pharmacodynamic (PD) pharmacokinetic (PK) and safety profile of formoterol delivered via two different dry different dry powder inhalers (DPIs) [Abstract]. European Respiratory Journal 2006; Vol. 28, issue Suppl 50:614s [E3606].
Dubakiene 2006 {published data only}
-
- Dubakiene R, Nargela R, Sakalauskas R, Vahteristo M, Silvasti M, Lahelma S. Clinically equivalent bronchodilatation achieved with formoterol delivered via Easyhaler and Aerolizer. Respiration 2006; Vol. 73, issue 4:441‐8. - PubMed
Dusser 2005 {published data only}
-
- Dusser D, Vicaut E, Lefrancois G. Double‐blind, double‐dummy, multinational, multicenter, parallel‐group design clinical trial of clinical non‐inferiority of formoterol 12 microg/unit dose in a b.i.d. regimen administered via an HFA‐propellant‐pMDI or a dry powder inhaler in a 12‐week treatment period of moderate to severe stable persistent asthma in adult patients. Respiration 2005; Vol. 72, issue Suppl 1:20‐7. - PubMed
Ericsson 2006 {published data only}
-
- Ericsson K, Bantje TA, Huber RM, Borg S, Bateman ED. Cost‐effectiveness analysis of budesonide/formoterol compared with fluticasone in moderate‐persistent asthma. Respiratory Medicine 2006; Vol. 100, issue 4:586‐94. - PubMed
Eryonucu 2005 {published data only}
-
- Eryonucu B, Uzun K, Guler N, Tuncer M, Sezgi C. Comparison of the short‐term effects of salmeterol and formoterol on heart rate variability in adult asthmatic patients. Chest 2005; Vol. 128, issue 3:1136‐9. - PubMed
Ferrari 2000 {published data only}
-
- Ferrari M, Balestreri F, Baratieri S, Biasin C, Oldani V, Lo Cascio V. Evidence of the rapid protective effect of formoterol dry‐powder inhalation against exercise‐induced bronchospasm in athletes with asthma. Respiration 2000; Vol. 67, issue 5:510‐3. - PubMed
Fitoussi 2002 {published data only}
-
- Fitoussi S, Dobson C, Ayre G, Langholff W. Safety and tolerability profile of high dose formoterol (Aerolizer®) and salbutamol (MDI) given for three days in patients with mild persistent asthma. Chest 2002:P117.
Gessner 2003 {published data only}
-
- Gessner C, Stenglein S, Brautigam M, Muller A, Schauer J. Miflonide/Foradil via Aerolizer compared with other anti‐inflammatory and anti‐obstructive therapeutic regimens [German]. Pneumologie 2003; Vol. 57, issue 3:137‐43. - PubMed
Graff‐Lonnevig 1990 {published data only}
-
- Graff‐Lonnevig V, Browaldh L. Twelve hours' bronchodilating effect of inhaled formoterol in children with asthma: a double‐blind cross‐over study versus salbutamol. Clinical and Experimental Allergy 1990; Vol. 20, issue 4:429‐32. - PubMed
Green 2006 {published data only}
-
- Green RH, Brightling CE, McKenna S, Hargadon B, Neale N, Parker D, et al. Comparison of asthma treatment given in addition to inhaled corticosteroids on airway inflammation and responsiveness. European Respiratory Journal 2006; Vol. 27, issue 6:1144‐51. - PubMed
Happonen 2009 {published data only}
-
- Happonen P, Harkonen M, Vaheri R, Rytila P. Long‐term safety of inhaled formoterol in adult asthma patients [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16 2009:[P1964].
Hedenstrom 1992 {published data only}
-
- Hedenstrom H, Wegener T, Boman G, Wahlander L, Melander B. Effect of inhaled formoterol versus terbutaline on respiratory function in moderate bronchial asthma. Allergy 1992; Vol. 47, issue 2 Pt 2:158‐63. - PubMed
Hermansen 2006 {published data only}
-
- Hermansen MN, Nielsen KG, Buchvald F, Jespersen JJ, Bengtsson T, Bisgaard H. Acute relief of exercise‐induced bronchoconstriction by inhaled formoterol in children with persistent asthma. Chest 2006; Vol. 129, issue 5:1203‐9. - PubMed
Houghton 2004 {published data only}
-
- Houghton CM, Langley SJ, Singh SD, Holden J, Monici Preti AP, Acerbi D, et al. Comparison of bronchoprotective and bronchodilator effects of a single dose of formoterol delivered by hydrofluoroalkane and chlorofluorocarbon aerosols and dry powder in a double blind, placebo‐controlled, crossover study. British Journal of Clinical Pharmacology 2004; Vol. 58, issue 4:359‐66. - PMC - PubMed
Ind 2002 {published data only}
-
- Ind PW, Villasante C, Shiner RJ, Pietinalho A, Boszormenyi NG, Soliman S, et al. Safety of formoterol by Turbuhaler as reliever medication compared with terbutaline in moderate asthma. European Respiratory Journal 2002; Vol. 20, issue 4:859‐66. - PubMed
Jain 2004 {published data only}
-
- Jain A, Raghuram J. Randomized controlled study of the safety and efficacy of PRN formoterol compared to PRN albuterol for the management of asthma [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando 2004:B36 Poster G17.
Jenkins 2005 {published data only}
-
- Jenkins CR, Thien FCK, Wheatley JR, Reddel HK. Traditional and patient‐centred outcomes with three classes of asthma medication. European Respiratory Journal 2005; Vol. 26, issue 1:36‐44. - PubMed
Kamenov 2007 {published data only}
-
- Kamenov A, Kamenov S, Kamenov B. The efficacy and safety formoterol‐HFA pMDI in school children with persistent asthma [Abstract]. European Respiratory Journal 2007; Vol. 30, issue Suppl 51:460s [E2712].
Kesten 1992 {published data only}
-
- Kesten S, Chapman KR, Broder I, Cartier A, Hyland RH, Knight A, et al. Sustained improvement in asthma with long‐term use of formoterol fumarate. Annals of Allergy 1992; Vol. 69, issue 5:415‐20. - PubMed
Kohler 2003 {published data only}
-
- Kohler D, Schmidt F, Wiese C, Dellweg D, Haidl P. Safety concerns regarding the use of the formoterol Turbohaler® for acute asthma attacks [abstract]. American Thoracic Society 99th International Conference 2003:B036 Poster H74.
Kruse 2005 {published data only}
-
- Kruse M, Rosenkranz B, Dobson C, Ayre G, Horowitz A. Safety and tolerability of high‐dose formoterol (Aerolizer) and salbutamol (pMDI) in patients with mild/moderate, persistent asthma. Pulmonary Pharmacology and Therapeutics 2005; Vol. 18, issue 3:229‐34. - PubMed
Lebecque 1994 {published data only}
-
- Lebecque P, Vliers S, Saint‐Moulin T, Godding V. The effect of a single dose of formoterol using an aerosol inhaler in asthmatic children. A randomized, controlled, double‐blind study. Revue des Maladies Respiratoires 1994; Vol. 11, issue 1:47‐50. - PubMed
Lebecque 1994a {published data only}
-
- Lebecque P, Vliers S, De SMT, Godding V. Response to a single dose of inhaled formoterol in asthmatic children: a double‐blind randomized controlled study. Revue des Maladies Respiratoires 1994; Vol. 11, issue 1:47‐50. - PubMed
Lee‐Wong 2008 {published data only}
-
- Lee‐Wong M, Chou V, Ogawa Y. Formoterol fumarate inhalation powder vs albuterol nebulizer for the treatment of asthma in the acute care setting. Annals of Allergy, Asthma, and Immunology 2008; Vol. 100, issue 2:146‐52. - PubMed
Lemaigre 2006 {published data only}
-
- Lemaigre V, Bergh O, Smets A, Peuter S, Verleden GM. Effects of long‐acting bronchodilators and placebo on histamine‐induced asthma symptoms and mild bronchus obstruction. Respiratory Medicine 2006; Vol. 100, issue 2:348‐53. - PubMed
Lotvall 1997 {published data only}
-
- Lotvall J, Persson G, Larsson P, Mardell C, Rosenborg J. Tolerability of inhaled high doses of formoterol and salbutamol in asthmatic patients maintained on twice daily formoterol 12 (9µg) delivered [abstract]. European Respiratory Journal. Supplement 1997; Vol. 10 Suppl 25:103S.
Lotvall 2005 {published data only}
-
- Lotvall J, Palmqvist M, Ankerst J, Persson G, Rosenborg J, Bengtsson T, et al. The effect of formoterol over 24 h in patients with asthma: the role of enantiomers. Pulmonary Pharmacology and Therapeutics 2005; Vol. 18, issue 2:109‐13. - PubMed
Lotvall 2008 {published data only}
-
- Lotvall J, Ankerst J. Long duration of airway but not systemic effects of inhaled formoterol in asthmatic patients. Respiratory Medicine 2008; Vol. 102, issue 3:449‐56. - PubMed
Lyseng‐Williamson 2003 {published data only}
-
- Lyseng‐Williamson KA, Plosker GL. Inhaled salmeterol/fluticasone propionate combination: a pharmacoeconomic review of its use in the management of asthma. Pharmacoeconomics 2003; Vol. 21, issue 13:951‐89. - PubMed
Maesen 1992 {published data only}
-
- Maesen FP, Costongs R, Smeets SJ, Zweers PG, Goedhart DM. Formoterol as dry powder inhalation. A dose finding study in comparison with formoterol metered dose inhaler and placebo. Chest 1992; Vol. 101, issue 5:1376‐81. - PubMed
Maesen 1992a {published data only}
-
- Maesen FP, Nakratzas G, Bantje TA, Prins J, Zweers PG. Formoterol suspension aerosol. Comparison with formoterol solution aerosol for 12 weeks in asthmatic patients. Chest 1992; Vol. 102, issue 5:1544‐9. - PubMed
Malo 1990 {published data only}
-
- Malo JL, Cartier A, Trudeau C, Ghezzo H, Gontovnick L. Formoterol, a new inhaled beta‐2 adrenergic agonist, has a longer blocking effect than albuterol on hyperventilation‐induced bronchoconstriction. American Review of Respiratory Disease 1990; Vol. 142, issue 5:1147‐52. - PubMed
Malolepszy 2001 {published data only}
-
- Malolepszy J, Boszormenyi Nagy G, Selroos O, Larsso P, Brander R. Safety of formoterol Turbuhaler at cumulative dose of 90 microg in patients with acute bronchial obstruction. European Respiratory Journal 2001; Vol. 18, issue 6:928‐34. - PubMed
Malolepszy 2002 {published data only}
-
- Malolepszy J. Efficacy and tolerability of oral theophylline slow‐release versus inhalative formoterol in moderate asthma poorly controlled on low‐dose steroids. Atemwegs‐ und Lungenkrankheiten 2002; Vol. 28, issue 2:78‐87.
Mann 2003 {published data only}
-
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003; Vol. 124, issue 1:70‐4. - PubMed
Marzo 2000 {published data only}
-
- Marzo A, Monti NC, Tettamanti RA, Crivelli F, Dal Bo L, Mazzucchelli P, et al. Bioequivalence of inhaled formoterol fumarate assessed from pharmacodynamic, safety and urinary pharmacokinetic data. Arzneimittel‐Forschung 2000; Vol. 50, issue 6:559‐63. - PubMed
Matthys 2004 {published data only}
-
- Matthys H, Behling B, Behling E. Comparison of formoterol (12 and 6 mug b.i.d) vs terbutaline (0.5 mg b.i.d) with equal doses of inhaled budesonide (0.2 mg bid) in 246 children with mild to moderate asthma. Atemwegs‐ und Lungenkrankheiten 2004; Vol. 30, issue 5:251‐6.
Midgren 1992 {published data only}
-
- Midgren B, Melander B, Persson G. Formoterol, a new long‐acting beta 2 agonist, inhaled twice daily, in stable asthmatic subjects. Chest 1992; Vol. 101, issue 4:1019‐22. - PubMed
Molimard 2005 {published data only}
-
- Molimard M, Guenole E, Duvauchelle T, Vicaut E, Lefrancois G. A randomized, double‐blind, double‐dummy, safety crossover trial comparing cumulative doses up to 96 microg of formoterol delivered via an HFA‐134a‐propelled pMDI vs. same cumulative doses of formoterol DPI and placebo in asthmatic patients. Respiration 2005; Vol. 72, issue Suppl 1:28‐34. - PubMed
Najafizadeh 2007 {published data only}
Nandeuil 2006 {published data only}
-
- Nandeuil A, Kottakis I, Raptis H, Roslan H, Ivanov Y, Woodcock A. Safety and tolerability of the novel very long acting B2 agonist carmoterol given as a 2µg qd dose 8 days comparison with formoterol and placebo in patients with persistent asthma [Abstract]. European Respiratory Journal 2006; Vol. 28, issue Suppl 50:665s [P3859].
Newnham 1994 {published data only}
-
- Newnham DM, McDevitt DG, Lipworth BJ. Bronchodilator subsensitivity after chronic dosing with eformoterol in patients with asthma. American Journal of Medicine 1994; Vol. 97, issue 1:29‐37. - PubMed
Newnham 1995 {published data only}
Novartis 2005b {published data only}
-
- Novartis. A 12‐month multicenter, randomized, double‐blind, double‐dummy trial to examine the long‐term tolerability of fomoterol 10µg via the multiple dose dry powder inhaler (MDDPI), both as twice daily maintenance therapy and as on‐demand use in addition to maintenance in patients with persistent asthma. http://pharma.us.novartis.com/ 2005.
Otto‐Knapp 2008 {published data only}
-
- Otto‐Knapp R, Conrad F, Hosch S, Metzenauer P, Maus J, Noga O, et al. Efficacy and safety of formoterol delivered through the Novolizer, a novel dry powder inhaler (DPI) compared with a standard DPI in patients with moderate to severe asthma. Pulmonary Pharmacology and Therapeutics 2008; Vol. 21, issue 1:47‐53. - PubMed
Patessio 1991 {published data only}
-
- Patessio A, Podda A, Carone M, Trombetta N, Donner CF. Protective effect and duration of action of formoterol aerosol on exercise‐induced asthma. European Respiratory Journal 1991; Vol. 4, issue 3:296‐300. - PubMed
Pauwels 2003 {published data only}
-
- Pauwels RA, Sears MR, Campbell M, Villasante C, Huang S, Lindh A, et al. Formoterol as relief medication in asthma: a worldwide safety and effectiveness trial. European Respiratory Journal 2003; Vol. 22, issue 5:787‐94. - PubMed
Pearlman 2002 {published data only}
-
- Pearlman DS, Kottakis J, Till D, Della Cioppa G. Formoterol delivered via a dry powder inhaler (Aerolizer): results from long‐term clinical trials in children. Current Medical Research and Opinion 2002; Vol. 18, issue 8:445‐55. - PubMed
Pohunek 2004 {published data only}
-
- Pohunek P, Matulka M, Rybnicek O, Kopriva F, Honomichlova H, Svobodova T. Dose‐related efficacy and safety of formoterol (Oxis) Turbuhaler compared with salmeterol Diskhaler in children with asthma. Pediatric Allergy & Immunology 2004; Vol. 15, issue 1:32‐9. - PubMed
Price 2002 {published data only}
Price 2008 {published data only}
-
- Price J, Radner F, Hultquist C, Lindberg B. Safety of formoterol in children and adolescents: experience from asthma clinical trials [Abstract]. European Respiratory Society Annual Congress, Berlin, Germany, October 4‐8 2008:[E3053].
Randell 2005 {published data only}
-
- Randell J, Saarinen A, Walamies M, Vahteristo M, Silvasti M, Lahelma S. Safety of formoterol after cumulative dosing via Easyhaler and Aerolizer. Respiratory Medicine 2005; Vol. 99, issue 12:1485‐93. - PubMed
Richter 2007 {published data only}
-
- Richter K, Hartmann U, Metzenauer P, Magnussen H. Randomised trial comparing as‐needed versus regular treatment with formoterol in patients with persistent asthma. Respiratory Medicine 2007; Vol. 101, issue 3:467‐75. - PubMed
Rico‐Mendez 1999 {published data only}
-
- Rico‐Mendez FG, Ochoa G, Rocio Chapela M, Diaz M, Cohen V, Ibarra J, et al. Dry powdered formoterol, twice a day versus aerosolized salbutamol, four times a day, in patients with stable asthma. Revista Alergia Mexico 1999; Vol. 46, issue 5:130‐5. - PubMed
Ringdal 1998 {published data only}
-
- Ringdal N, Derom E, Wahlin‐Boll E, Pauwels R. Onset and duration of action of single doses of formoterol inhaled via Turbuhaler. Respiratory Medicine 1998; Vol. 92, issue 8:1017‐21. - PubMed
Rosenborg 2000 {published data only}
-
- Rosenborg J, Bengtsson T, Larsson P, Blomgren A, Persson G, Lötvall J. Relative systemic dose potency and tolerability of inhaled formoterol and salbutamol in healthy subjects and asthmatics. European Journal of Clinical Pharmacology 2000; Vol. 56, issue 5:363‐70. - PubMed
Rosenborg 2002 {published data only}
-
- Rosenborg J, Bengtsson T, Larsson P, Blomgren A, Persson G, Lötvall J. Relative systemic dose potency and tolerability of inhaled formoterol and salbutamol in healthy subjects and asthmatics. European Journal of Clinical Pharmacology 2002; Vol. 58, issue 4:S53‐60. - PubMed
Rosenborg 2002a {published data only}
-
- Rosenborg J, Larsson P, Rott Z, Bocskei C, Poczi M, Juhasz G. Assessment of a relative therapeutic index between inhaled formoterol and salbutamol in asthma patients. European Journal of Clinical Pharmacology 2002; Vol. 58, issue 4:S61‐7. - PubMed
Rubinfeld 2006 {published data only}
-
- Rubinfeld AR, Scicchitano R, Hunt A, Thompson PJ, Nooten A, Selroos O. Formoterol Turbuhaler as reliever medication in patients with acute asthma. European Respiratory Journal. 2006; Vol. 27, issue 4:735‐41. - PubMed
Schlimmer 2002 {published data only}
-
- Schlimmer P. Single‐dose comparison of formoterol (Oxis) Turbuhaler 6 mug and formoterol Aerolizer 12 mug in moderate to severe asthma: a randomised, crossover study. Pulmonary Pharmacology and Therapeutics 2002; Vol. 15, issue 4:369‐74. - PubMed
Sprogoe 1992 {published data only}
-
- Sprogoe Jakobsen U, Viktrup L, Davidsen O, Viskum K. Bronchial asthma treated with long‐acting beta 2 agonist. Comparison between formoterol 12 mu/g inhaled twice daily and salbutamol 200. Ugeskrift for Laeger 1992; Vol. 154, issue 47:3325‐8. - PubMed
Stahl 2003 {published data only}
-
- Stahl E, Postma DS, Svensson K, Tattersfield AE, Eivindson A, Schreurs A, et al. Formoterol used as needed improves health‐related quality of life in asthmatic patients uncontrolled with inhaled corticosteroids. Respiratory Medicine 2003; Vol. 97, issue 9:1061‐6. - PubMed
Stelmach 2008 {published data only}
-
- Stelmach I, Grzelewski T, Majak P, Jerzynska J, Stelmach W, Kuna P. Effect of different antiasthmatic treatments on exercise‐induced bronchoconstriction in children with asthma. Journal of Allergy & Clinical Immunology 2008; Vol. 121, issue 2:383‐9. - PubMed
Tattersfield 1999 {published data only}
-
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. American Journal of Respiratory and Critical Care Medicine 1999; Vol. 160, issue 2:594‐9. - PubMed
Tattersfield 2001 {published data only}
-
- Tattersfield AE, Lofdahl CG, Postma DS, Eivindson A, Schreurs AG, Rasidakis A, et al. Comparison of formoterol and terbutaline for as‐needed treatment of asthma: a randomised trial. Lancet 2001; Vol. 357, issue 9252:257‐61. - PubMed
Totterman 1998 {published data only}
-
- Totterman KJ, Huhti L, Sutinen E, Backman R, Pietinalho A, Falck M, et al. Tolerability to high doses of formoterol and terbutaline via Turbuhaler for 3 days in stable asthmatic patients. European Respiratory Journal 1998; Vol. 12, issue 3:573‐9. - PubMed
van den Berg 1995 {published data only}
-
- Berg BT, Smeets JJ, Boxtel CJ, Maesen FP. Evaluation of different doses of formoterol from a newly developed powder inhalation device in asthmatic patients. Fundamental & Clinical Pharmacology 1995; Vol. 9, issue 6:593‐603. - PubMed
van der Woude 2004 {published data only}
-
- Woude HJ, Postma DS, Politiek MJ, Winter TH, Aalbers R. Relief of dyspnoea by beta2‐agonists after methacholine‐induced bronchoconstriction. Respiratory Medicine 2004; Vol. 98, issue 9:816‐20. - PubMed
van Veen 2003 {published data only}
-
- Veen A, Weller FR, Wierenga EA, Jansen HM, Jonkers RE. A comparison of salmeterol and formoterol in attenuating airway responses to short‐acting beta2‐agonists. Pulmonary Pharmacology and Therapeutics 2003; Vol. 16, issue 3:153‐61. - PubMed
Verini 1998 {published data only}
-
- Verini M, Verrotti A, Greco R, Chiarelli F. Comparison of the bronchodilator effect of inhaled short‐ and long‐acting beta2‐agonists in children with bronchial asthma. A randomised trial. Clinical Drug Investigation 1998; Vol. 16, issue 1:19‐24. - PubMed
Villa 2002 {published data only}
-
- Villa J, Kuna P, Egner J, Brander R. A 6‐month comparison of the safety profiles of formoterol (Oxis®) Turbuhaler® as needed and terbutaline (Bricanyl®) Turbuhaler® as needed in asthmatic children on anti‐inflammatory medication [abstract]. American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A746.
Villa 2002a {published data only}
-
- Villa J, Kuna P, Egner J, Brander R. Safety of formoterol reliever therapy compared with terbutaline in asthmatic children taking anti‐inflammatory therapy [abstract]. European Respiratory Society Annual Congress 2002 2002:Abstract no: P2739.
Vilsvik 2001 {published data only}
-
- Vilsvik J, Ankerst J, Palmqvist M, Persson G, Schaanning J, Schwabe G, et al. Protection against cold air and exercise‐induced bronchoconstriction while on regular treatment with Oxis. Respiratory Medicine 2001; Vol. 95, issue 6:484‐90. - PubMed
Wallin 1999 {published data only}
-
- Wallin A, Sandstrom T, Soderberg M, Howarth P, Lundback B, Della‐Cioppa G, et al. The effects of regular inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indices in mild asthma. American Journal of Respiratory and Critical Care Medicine 1999; Vol. 159, issue 1:79‐86. - PubMed
Wegener 1992 {published data only}
-
- Wegener T, Hedenstrom H, Melander B. Rapid onset of action of inhaled formoterol in asthmatic patients. Chest 1992; Vol. 102, issue 2:535‐8. - PubMed
Wong 1992 {published data only}
-
- Wong BJ, Dolovich J, Ramsdale EH, O'Byrne P, Gontovnick L, Denburg JA, et al. Formoterol compared with beclomethasone and placebo on allergen‐induced asthmatic responses. American Review of Respiratory Disease 1992; Vol. 146, issue 5 Pt 1:1156‐60. - PubMed
Yasuo 1984 {published data only}
-
- Yasuo K, TakaoI S, Mitsuru W, Terumi T, Shigenori N, Eisei N, et al. Evaluation of therapeutic effects of formoterol, a new beta‐receptor stimulating bronchodilator, on bronchial asthma ‐ a joint double‐blind repeated‐dose study at 37 institutions. Rinsho Hyoka (Clinical Evaluation) 1984; Vol. 12, issue 3:697‐727.
Yasuo 1984a {published data only}
-
- Yasuo K, Terumi T, Mitsuru I, Takao S, Shigenori N, Eisei N, et al. Evaluation of therapeutic effects of formoterol, a beta‐receptor stimulating bronchodilator, on bronchial asthma ‐ a joint double‐blind single‐dose study at 37 institutions Part 2. Rinsho Hyoka (Clinical Evaluation) 1984; Vol. 12, issue 3:671‐95.
Yasuo 1984b {published data only}
-
- Yasuo K, TerumiI T, Kazuhiko TO, Takao S, Shigenori N, Eisei N, et al. Evaluation of therapeutic effects of formoterol, a beta‐receptor stimulating bronchodilator, on bronchial asthma ‐ a joint double‐blind single‐dose study at 30 institutions Part 1. Rinsho Hyoka (Clinical Evaluation) 1984; Vol. 12, issue 3:647‐70.
Yates 1995 {published data only}
-
- Yates DH, Sussman HS, Shaw MJ, Barnes PJ, Chung KF. Regular formoterol treatment in mild asthma: effect on bronchial responsiveness during and after treatment. American Journal of Respiratory and Critical Care Medicine 1995; Vol. 152, issue 4 I:1170‐4. - PubMed
Yurdakul 2002 {published data only}
-
- Yurdakul AS, Calisir HC, Tunctan B, Ogretensoy M. Comparison of second controller medications in addition to inhaled corticosteroid in patients with moderate asthma. Respiratory Medicine 2002; Vol. 96, issue 5:322‐9. - PubMed
Additional references
Altman 2003
Anderson 2006
-
- Anderson GP. Current issues with beta(2)‐adrenoceptor agonists ‐ pharmacology and molecular and cellular mechanisms. Clinical Reviews in Allergy and Immunology 2006; Vol. 31, issue 2‐3:119‐30. - PubMed
Arnold 1985
-
- Arnold JMO, O'Connor PC, Riddell JG, Harron DWG, Shanks RG, McDevitt DG. Effects of the beta‐2‐adrenoceptor antagonist ici‐118,551 on exercise tachycardia and isoprenaline‐induced beta‐adrenoceptor responses in man. British Journal of Clinical Pharmacology 1985; Vol. 19, issue 5:619‐30. - PMC - PubMed
Barnes 1993
-
- Barnes PJ. Beta‐adrenoceptors on smooth‐muscle, nerves and inflammatory cells. Life Sciences 1993; Vol. 52, issue 26:2101‐9. - PubMed
Barnes 1995
-
- Barnes PJ. Beta‐adrenergic receptors and their regulation. American Journal of Respiratory and Critical Care Medicine 1995; Vol. 152, issue 3:838‐60. - PubMed
Bennett 1994
Benson 1948
-
- Benson RL, Perlman F. Clinical effects of epinephrine by inhalation ‐ a survey. Journal of Allergy 1948; Vol. 19, issue 2:129‐40. - PubMed
Bergman 1969
-
- Bergman J, Persson H, Wetterlin K. Two new groups of selective stimulants of adrenergic beta‐receptors. Experientia 1969; Vol. 25, issue 9:899‐901. - PubMed
Bijl‐Hofland 2001
-
- Bijl‐Hofland ID, Cloosterman SG, Folgering HT, Elshout FJ, Weel C, Schayck CP. Inhaled corticosteroids, combined with long‐acting beta(2)‐agonists, improve the perception of bronchoconstriction in asthma. American Journal of Respiratory and Critical Care Medicine 2001; Vol. 164, issue 5:764‐9. - PubMed
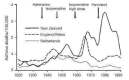
Blauw 1995
-
- Blauw GJ, Westendorp RGJ. Asthma deaths in New Zealand ‐ whodunnit. The Lancet 1995;345:2‐3. - PubMed
Boushey 1980
-
- Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. American Review of Respiratory Disease 1980; Vol. 121, issue 2:389‐413. [(Print)] - PubMed
Bousquet 1990
-
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. New England Journal of Medicine 1990; Vol. 323, issue 15:1033‐9. - PubMed
Bousquet 2000
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. American Journal Respiratory Critical Care Medicine 2000; Vol. 161, issue 5:1720‐45. - PubMed
Bradburn 2007
-
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007; Vol. 26, issue 1:53‐77. [0277‐6715: (Print)] - PubMed
Brewster 1990
-
- Brewster CEP, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial‐asthma. American Journal of Respiratory Cell and Molecular Biology 1990; Vol. 3, issue 5:507‐11. - PubMed
Brown 1983
-
- Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta‐2‐receptor stimulation by circulating epinephrine. New England Journal of Medicine 1983; Vol. 309, issue 23:1414‐9. - PubMed
Brusasco 1998
Burgess 1991
-
- Burgess CD, Windom HH, Pearce N, Marshall S, Beasley R, Siebers RWL, et al. Lack of evidence for beta‐2 receptor selectivity ‐ a study of metaproterenol, fenoterol, isoproterenol, and epinephrine in patients with asthma. American Review of Respiratory Disease 1991; Vol. 143, issue 2:444‐6. - PubMed
Burggraaf 2001
Carroll 1993
-
- Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. American Review of Respiratory Disease 1993; Vol. 147, issue 2:405‐10. - PubMed
Cates 2008
Cates 2009
Cates 2009a
Cates 2011
-
- Cates CJ, Lasserson TJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD007694.pub2] - DOI - PMC - PubMed
Cockcroft 1993
-
- Cockcroft DW, McParland CP, Britto SA, Swystun VA, Rutherford BC. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet 1993; Vol. 342, issue 8875:833‐7. - PubMed
Cockcroft 2006
-
- Cockcroft D. Airway hyperresponsiveness as a determinant of the early asthmatic response to inhaled allergen. Journal of Asthma 2006; Vol. 43, issue 3:175‐8. - PubMed
Collins 1969
Crane 1989
-
- Crane J, Pearce N, Flatt A, Burgess C, Jackson R, Kwong T, et al. Prescribed fenoterol and death from asthma in New Zealand, 1981‐83 ‐ case‐control study. Lancet 1989; Vol. 1, issue 8644:917‐22. - PubMed
Cullum 1969
Ducharme 2006
Dunnill 1960
Ebina 1993
-
- Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial‐asthma ‐ a 3‐d morphometric study. American Review of Respiratory Disease 1993; Vol. 148, issue 3:720‐6. - PubMed
Gay 1949
-
- Gay LN, Long JW. Clinical evaluation of isopropylepinephrine in management of bronchial asthma. JAMA 1949; Vol. 139, issue 7:452‐7. - PubMed
Grainger 1991
Greenstone 2005
-
- Greenstone IR, Ni Chroinin M, Masse V, Danish A, Magdalinos H, Zhang X, et al. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005533] - DOI - PubMed
Guhan 2000
Haley 1998
-
- Haley KJ, Drazen JM. Inflammation and airway function in asthma ‐ what you see is not necessarily what you get. American Journal of Respiratory and Critical Care Medicine 1998; Vol. 157, issue 1:1‐3. - PubMed
Hall 1989
-
- Hall JA, Petch MC, Brown MJ. Intracoronary injections of salbutamol demonstrate the presence of functional beta‐2‐adrenoceptors in the human‐heart. Circulation Research 1989; Vol. 65, issue 3:546‐53. - PubMed
Hanania 2002
-
- Hanania NA, Sharfkhaneh A, Barber R, Dickey BF. Beta‐agonist intrinsic efficacy ‐ measurement and clinical significance. American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165, issue 10:1353‐8. - PubMed
Hanania 2007
-
- Hanania NA, Moore RH, Zimmerman JL, Miller CT, Bag R, Sharafkhaneh A, et al. The role of intrinsic efficacy in determining response to beta(2)‐agonist in acute severe asthma. Respiratory Medicine 2007; Vol. 101, issue 5:1007‐14. - PubMed
Hancox 1999
-
- Hancox RJ, Aldridge RE, Cowan JO, Flannery EM, Herbison GP, McLachlan CR, et al. Tolerance to beta‐agonists during acute bronchoconstriction. European Respiratory Journal 1999; Vol. 14, issue 2:283‐7. - PubMed
Hancox 2006
-
- Hancox RJ. Interactions between corticosteroids and beta2‐agonists. Clinical Reviews in Allergy & Immunology 2006; Vol. 31, issue 2‐3:231‐46. [(Print)] - PubMed
Haney 2006
-
- Haney S, Hancox RJ. Recovery from bronchoconstriction and bronchodilator tolerance. Clinical Reviews in Allergy and Immunology 2006; Vol. 31, issue 2‐3:181‐96. - PubMed
Harvey 1982
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2008.
ICHE2a 1995
-
- Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Clinical safety data management: definitions and standards for expedited reporting. http://www.fda.gov/cder/guidance/iche2a.pdf 1995.
ICHE3 2007
-
- Expert working group (efficacy) of the international conference on harmonization of technical requirements for registration of pharmaceuticals for human use. FDA Center for Drug Evaluation and Research. Guideline for Industry Structure and Content of Clinical Study Reports. http://www.fda.gov/cder/guidance/iche3.pdf 25 August 2007.
Jones 2001
-
- Jones SL, Cowan JO, Flannery EM, Hancox RJ, Herbison GP, Taylor DR. Reversing acute bronchoconstriction in asthma: the effect of bronchodilator tolerance after treatment with formoterol. European Respiratory Journal 2001; Vol. 17, issue 3:368‐73. - PubMed
Kemp 1993
-
- Kemp JP, Bierman CW, Cocchetto DM. Dose‐response study of inhaled salmeterol in asthmatic‐patients with 24‐hour spirometry and Holter monitoring. Annals of Allergy 1993; Vol. 70, issue 4:316‐22. - PubMed
Konzett 1940
-
- Konzett H. Neue broncholytisch hochwirksame Körper der Adrenalinreihe. Naunyn‐Schmiedeberg's Archives of Pharmacology 1940; Vol. 197:27‐40.
Kuyper 2003
-
- Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, et al. Characterization of airway plugging in fatal asthma. American Journal of Medicine 2003; Vol. 115, issue 1:6‐11. [(Print)] - PubMed
Lee 2003
Lipworth 1989
-
- Lipworth BJ, Struthers AD, McDevitt DG. Tachyphylaxis to systemic but not to airway responses during prolonged therapy with high‐dose inhaled salbutamol in asthmatics. American Review of Respiratory Disease 1989; Vol. 140, issue 3:586‐92. - PubMed
Lipworth 1992
Lipworth 1997
-
- Lipworth BJ. Airway subsensitivity with long‐acting beta 2‐agonists: is there cause for concern?. Drug Safety 1997;16(5):295‐308. - PubMed
Lipworth 2000
-
- Lipworth BJ, Aziz I. Bronchodilator response to albuterol after regular formoterol and effects of acute corticosteroid administration. Chest 2000; Vol. 117, issue 1:156‐62. - PubMed
McDevitt 1974
McIvor 1998
-
- McIvor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory and Critical Care Medicine 1998; Vol. 158, issue 3:924‐30. - PubMed
McMahon 2011
Miranda 2004
-
- Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. Journal of Allergy and Clinical Immunology 2004; Vol. 113, issue 1:101‐8. [0091‐6749] - PubMed
Morrison 1993
-
- Morrison KJ, Gao Y, Vanhoutte PM. Beta‐adrenoceptors and the epithelial layer in airways. Life Sciences 1993; Vol. 52, issue 26:2123‐30. - PubMed
Nelson 1977
-
- Nelson HS, Raine D, Doner HC, Posey WC. Subsensitivity to bronchodilator action of albuterol produced by chronic administration. American Review of Respiratory Disease 1977; Vol. 116, issue 5:871‐8. - PubMed
Ni Chroinin 2004
Ni Chroinin 2005
-
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] - DOI - PubMed
NovartisNDA20‐831 2005
-
- Novartis. Foradil® Aerolizer® (formoterol fumarate inhalation powder) 12 mcg. NDA 20‐831. Appendix 1 – List of pre‐defined terms. http://www.fda.gov/OHRMS/DOCKETS/ac/05/briefing/2005‐4148B1_02_02‐Novart... Accessed 4 June 2008.
Ordonez 2001
-
- Ordonez CL, Khashayar R, Wong HH, Ferrando RON, Wu R, Hyde DM, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. American Journal of Respiratory and Critical Care Medicine 2001; Vol. 163, issue 2:517‐23. - PubMed
Palmqvist 1999
-
- Palmqvist M, Ibsen T, Mellen A, Lotvall J. Comparison of the relative efficacy of formoterol and salmeterol in asthmatic patients. American Journal of Respiratory and Critical Care Medicine 1999; Vol. 160, issue 1:244‐9. - PubMed
Pearce 1990
Pearce 2001
-
- Pearce N BR, Crane J, Burgess C. Epidemiology of asthma mortality. Asthma and Rhinitis. Oxford: Blackwell Scientific, 2001:56‐69.
Pearce 2007
-
- Pearce N. Adverse Reactions: the Fenoterol Story. 1st Edition. Auckland: Auckland University Press, 2007:215. [9781869403744]
Phillips 1990
-
- Phillips GD, Finnerty JP, Holgate ST. Comparative protective effect of the inhaled beta‐2‐agonist salbutamol (albuterol) on bronchoconstriction provoked by histamine, methacholine, and adenosine 5'‐monophosphate in asthma. Journal of Allergy and Clinical Immunology 1990; Vol. 85, issue 4:755‐62. - PubMed
Salpeter 2006
-
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Annals of Internal Medicine 2006;144(12):904‐12. - PubMed
Sears 1990
-
- Sears MR, Taylor DR, Print CG, Lake DC, Li QQ, Flannery EM, et al. Regular inhaled beta‐agonist treatment in bronchial‐asthma. Lancet 1990; Vol. 336, issue 8728:1391‐6. - PubMed
Simonsson 1967
SMART 2006
-
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, the SMART Study Group. The salmeterol multicenter asthma research trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129:15‐26. - PubMed
Speizer 1968
Stolley 1972
-
- Stolley PD. Asthma mortality ‐ why the United States was spared an epidemic of deaths due to asthma. American Review of Respiratory Disease 1972; Vol. 105, issue 6:883‐90. - PubMed
Tattersfield 2006
-
- Tattersfield AE. Current issues with beta(2)‐adrenoceptor agonists ‐ historical background. Clinical Reviews in Allergy and Immunology 2006; Vol. 31, issue 2‐3:107‐17. - PubMed
Tonnel 2001
-
- Tonnel AB, Gosset P, Tillie‐Leblond I. Characteristics of the inflammatory response in bronchial lavage fluids from patients with status asthmaticus. International Archives of Allergy and Immunology 2001; Vol. 124, issue 1‐3:267‐71. - PubMed
van der Woude 2001
Van Noord 1996
-
- Noord JA, Smeets JJ, Raaijmakers JAM, Bommer AM, Maesen FPV. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. European Respiratory Journal 1996; Vol. 9, issue 8:1684‐8. - PubMed
Vignola 1993
-
- Vignola AM, Campbell AM, Chanez P, Bousquet J, Paullacoste P, Michel FB, et al. HLA‐DR and ICAM‐1 expression on bronchial epithelial‐cells in asthma and chronic bronchitis. American Review of Respiratory Disease 1993; Vol. 148, issue 3:689‐94. - PubMed
Walters 2002
Walters 2007
-
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001385.pub2] - DOI - PMC - PubMed
Weber 1982
-
- Weber RW, Smith JA, Nelson HS. Aerosolized terbutaline in asthmatics ‐ development of subsensitivity with long‐term administration. Journal of Allergy and Clinical Immunology 1982; Vol. 70, issue 6:417‐22. - PubMed
Wiggs 1990
-
- Wiggs BR, Moreno R, Hogg JC, Hilliam C, Pare PD. A model of the mechanics of airway narrowing. Journal of Applied Physiology 1990; Vol. 69, issue 3:849‐60. - PubMed
Wilson 1981
-
- Wilson JD, Sutherland DC, Thomas AC. Has the change to beta‐agonists combined with oral theophylline increased cases of fatal asthma. Lancet 1981; Vol. 1, issue 8232:1235‐7. - PubMed
Wong 1990
-
- Wong CS, Pavord ID, Williams J, Britton JR, Tattersfield AE. Bronchodilator, cardiovascular, and hypokalemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet 1990; Vol. 336, issue 8728:1396‐9. - PubMed
Woodruff 2004
-
- Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. American Journal of Respiratory and Critical Care Medicine 2004; Vol. 169, issue 9:1001‐6. - PubMed
Yates 1996
-
- Yates DH, Kharitonov SA, Barnes PJ. An inhaled glucocorticoid does not prevent tolerance to the bronchoprotective effect of a long‐acting inhaled beta(2)‐agonist. American Journal of Respiratory and Critical Care Medicine 1996; Vol. 154, issue 6:1603‐7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

