Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites
- PMID: 22514276
- PMCID: PMC3370170
- DOI: 10.1074/jbc.M111.322537
Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites
Abstract
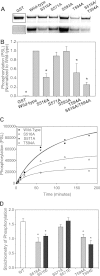
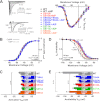
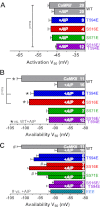
The cardiac Na(+) channel Na(V)1.5 current (I(Na)) is critical to cardiac excitability, and altered I(Na) gating has been implicated in genetic and acquired arrhythmias. Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) is up-regulated in heart failure and has been shown to cause I(Na) gating changes that mimic those induced by a point mutation in humans that is associated with combined long QT and Brugada syndromes. We sought to identify the site(s) on Na(V)1.5 that mediate(s) the CaMKII-induced alterations in I(Na) gating. We analyzed both CaMKII binding and CaMKII-dependent phosphorylation of the intracellularly accessible regions of Na(V)1.5 using a series of GST fusion constructs, immobilized peptide arrays, and soluble peptides. A stable interaction between δ(C)-CaMKII and the intracellular loop between domains 1 and 2 of Na(V)1.5 was observed. This region was also phosphorylated by δ(C)-CaMKII, specifically at the Ser-516 and Thr-594 sites. Wild-type (WT) and phosphomutant hNa(V)1.5 were co-expressed with GFP-δ(C)-CaMKII in HEK293 cells, and I(Na) was recorded. As observed in myocytes, CaMKII shifted WT I(Na) availability to a more negative membrane potential and enhanced accumulation of I(Na) into an intermediate inactivated state, but these effects were abolished by mutating either of these sites to non-phosphorylatable Ala residues. Mutation of these sites to phosphomimetic Glu residues negatively shifted I(Na) availability without the need for CaMKII. CaMKII-dependent phosphorylation of Na(V)1.5 at multiple sites (including Thr-594 and Ser-516) appears to be required to evoke loss-of-function changes in gating that could contribute to acquired Brugada syndrome-like effects in heart failure.
Figures




References
-
- Ruan Y., Liu N., Priori S. G. (2009) Sodium channel mutations and arrhythmias. Nat. Rev. Cardiol. 6, 337–348 - PubMed
-
- Veldkamp M. W., Viswanathan P. C., Bezzina C., Baartscheer A., Wilde A. A., Balser J. R. (2000) Two distinct congenital arrhythmias evoked by a multidysfunctional Na+ channel. Circ. Res. 86, E91–E97 - PubMed
-
- Abriel H. (2007) Roles and regulation of the cardiac sodium channel NaV1.5. Recent insights from experimental studies. Cardiovasc. Res. 76, 381–389 - PubMed
-
- Hoch B., Meyer R., Hetzer R., Krause E. G., Karczewski P. (1999) Identification and expression of δ-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ. Res. 84, 713–721 - PubMed
-
- Kirchhefer U., Schmitz W., Scholz H., Neumann J. (1999) Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc. Res. 42, 254–261 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

