Regulation of neuronal mRNA translation by CaM-kinase I phosphorylation of eIF4GII
- PMID: 22514323
- PMCID: PMC3346851
- DOI: 10.1523/JNEUROSCI.0030-12.2012
Regulation of neuronal mRNA translation by CaM-kinase I phosphorylation of eIF4GII
Abstract
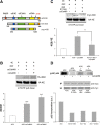
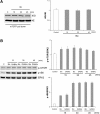
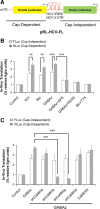
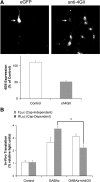
Ca²⁺/calmodulin-dependent kinases (CaMKs) are essential for neuronal development and plasticity, processes requiring de novo protein synthesis. Roles for CaMKs in modulating gene transcription are well established, but their involvement in mRNA translation is evolving. Here we report that activity-dependent translational initiation in cultured rat hippocampal neurons is enhanced by CaMKI-mediated phosphorylation of Ser1156 in eukaryotic initiation factor eIF4GII (4GII). Treatment with bicuculline or gabazine to enhance neuronal activity promotes recruitment of wild-type 4GII, but not the 4GII S1156A mutant or 4GI, to the heterotrimeric eIF4F (4F) complex that assembles at the 5' cap structure (m⁷GTP) of mRNA to initiate ribosomal scanning. Recruitment of 4GII to 4F is suppressed by pharmacological inhibition (STO-609) of CaM kinase kinase, the upstream activator of CaMKI. Post hoc in vitro CaMKI phosphorylation assays confirm that activity promotes phosphorylation of S1156 in transfected 4GII in neurons. Changes in cap-dependent and cap-independent translation were assessed using a bicistronic luciferase reporter transfected into neurons. Activity upregulates cap-dependent translation, and RNAi knockdown of CaMKIβ and γ isoforms, but not α or δ, led to its attenuation as did blockade of NMDA receptors. Furthermore, RNAi knockdown of 4GII attenuates cap-dependent translation and reduces density of dendritic filopodia and spine formation without effect on dendritic arborization. Together, our results provide a mechanistic link between Ca²⁺ influx due to neuronal activity and regulation of cap-dependent RNA translation via CaMKI activation and selective recruitment of phosphorylated 4GII to the 4F complex, which may function to regulate activity-dependent changes in spine density.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
