Initial HIV-1 antigen-specific CD8+ T cells in acute HIV-1 infection inhibit transmitted/founder virus replication
- PMID: 22514337
- PMCID: PMC3393529
- DOI: 10.1128/JVI.00437-12
Initial HIV-1 antigen-specific CD8+ T cells in acute HIV-1 infection inhibit transmitted/founder virus replication
Abstract
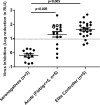
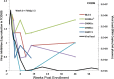
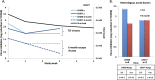
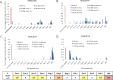
CD8-mediated virus inhibition can be detected in HIV-1-positive subjects who naturally control virus replication. Characterizing the inhibitory function of CD8(+) T cells during acute HIV-1 infection (AHI) can elucidate the nature of the CD8(+) responses that can be rapidly elicited and that contribute to virus control. We examined the timing and HIV-1 antigen specificity of antiviral CD8(+) T cells during AHI. Autologous and heterologous CD8(+) T cell antiviral functions were assessed longitudinally during AHI in five donors from the CHAVI 001 cohort using a CD8(+) T cell-mediated virus inhibition assay (CD8 VIA) and transmitted/founder (T/F) viruses. Potent CD8(+) antiviral responses against heterologous T/F viruses appeared during AHI at the first time point sampled in each of the 5 donors (Fiebig stages 1/2 to 5). Inhibition of an autologous T/F virus was durable to 48 weeks; however, inhibition of heterologous responses declined concurrent with the resolution of viremia. HIV-1 viruses from 6 months postinfection were more resistant to CD8(+)-mediated virus inhibition than cognate T/F viruses, demonstrating that the virus escapes early from CD8(+) T cell-mediated inhibition of virus replication. CD8(+) T cell antigen-specific subsets mediated inhibition of T/F virus replication via soluble components, and these soluble responses were stimulated by peptide pools that include epitopes that were shown to drive HIV-1 escape during AHI. These data provide insights into the mechanisms of CD8-mediated virus inhibition and suggest that functional analyses will be important for determining whether similar antigen-specific virus inhibition can be induced by T cell-directed vaccine strategies.
Figures




References
-
- Cao J, McNevin J, Malhotra U, McElrath MJ. 2003. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J. Immunol. 171:3837–3846 - PubMed
-
- Chakraborty H, et al. 2001. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 15:621–627 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI27767/AI/NIAID NIH HHS/United States
- U01 AI067854/AI/NIAID NIH HHS/United States
- R01A5779/PHS HHS/United States
- R24 DK064400/DK/NIDDK NIH HHS/United States
- P30 AI 64518/AI/NIAID NIH HHS/United States
- U19 AI067854/AI/NIAID NIH HHS/United States
- R24 DK-64400/DK/NIDDK NIH HHS/United States
- MC_U137884177/MRC_/Medical Research Council/United Kingdom
- P30 AI064518/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- R01 TW009237/TW/FIC NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

