Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense
- PMID: 22514521
- PMCID: PMC3324116
- DOI: 10.3389/fnana.2012.00009
Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense
Abstract
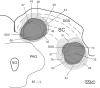
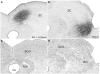
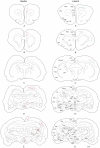
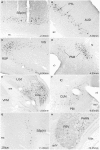
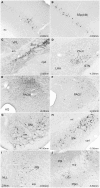
The superior colliculus (SC) is responsible for sensorimotor transformations required to direct gaze toward or away from unexpected, biologically salient events. Significant changes in the external world are signaled to SC through primary multisensory afferents, spatially organized according to a retinotopic topography. For animals, where an unexpected event could indicate the presence of either predator or prey, early decisions to approach or avoid are particularly important. Rodents' ecology dictates predators are most often detected initially as movements in upper visual field (mapped in medial SC), while appetitive stimuli are normally found in lower visual field (mapped in lateral SC). Our purpose was to exploit this functional segregation to reveal neural sites that can bias or modulate initial approach or avoidance responses. Small injections of Fluoro-Gold were made into medial or lateral sub-regions of intermediate and deep layers of SC (SCm/SCl). A remarkable segregation of input to these two functionally defined areas was found. (i) There were structures that projected only to SCm (e.g., specific cortical areas, lateral geniculate and suprageniculate thalamic nuclei, ventromedial and premammillary hypothalamic nuclei, and several brainstem areas) or SCl (e.g., primary somatosensory cortex representing upper body parts and vibrissae and parvicellular reticular nucleus in the brainstem). (ii) Other structures projected to both SCm and SCl but from topographically segregated populations of neurons (e.g., zona incerta and substantia nigra pars reticulata). (iii) There were a few brainstem areas in which retrogradely labeled neurons were spatially overlapping (e.g., pedunculopontine nucleus and locus coeruleus). These results indicate significantly more structures across the rat neuraxis are in a position to modulate defense responses evoked from SCm, and that neural mechanisms modulating SC-mediated defense or appetitive behavior are almost entirely segregated.
Keywords: approach; defense; segregated anatomical inputs; superior colliculus.
Figures





Similar articles
-
Topographical organization of the nigrotectal projection in rat: evidence for segregated channels.Neuroscience. 1992 Oct;50(3):571-95. doi: 10.1016/0306-4522(92)90448-b. Neuroscience. 1992. PMID: 1279464
-
Afferent projections to the superior colliculus in the rat, with special attention to the deep layers.J Hirnforsch. 1985;26(6):667-81. J Hirnforsch. 1985. PMID: 4093595
-
Multisensory processing via early cortical stages: Connections of the primary auditory cortical field with other sensory systems.Neuroscience. 2006 Dec 28;143(4):1065-83. doi: 10.1016/j.neuroscience.2006.08.035. Epub 2006 Oct 4. Neuroscience. 2006. PMID: 17027173
-
Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus.Physiol Rev. 1986 Jan;66(1):118-71. doi: 10.1152/physrev.1986.66.1.118. Physiol Rev. 1986. PMID: 3511480 Review.
-
Organization of subcortical pathways for sensory projections to the limbic cortex. II. Afferent projections to the thalamic lateral dorsal nucleus in the rat.J Comp Neurol. 1987 Nov 8;265(2):189-202. doi: 10.1002/cne.902650204. J Comp Neurol. 1987. PMID: 3320109 Review.
Cited by
-
Defense-like behaviors evoked by pharmacological disinhibition of the superior colliculus in the primate.J Neurosci. 2013 Jan 2;33(1):150-5. doi: 10.1523/JNEUROSCI.2924-12.2013. J Neurosci. 2013. PMID: 23283329 Free PMC article.
-
Different strategies in pointing tasks and their impact on clinical bedside tests of spatial orientation.J Neurol. 2022 Nov;269(11):5738-5745. doi: 10.1007/s00415-022-11015-z. Epub 2022 Mar 8. J Neurol. 2022. PMID: 35258851 Free PMC article.
-
Segregated fronto-cortical and midbrain connections in the mouse and their relation to approach and avoidance orienting behaviors.J Comp Neurol. 2017 Jun 1;525(8):1980-1999. doi: 10.1002/cne.24186. Epub 2017 Mar 20. J Comp Neurol. 2017. PMID: 28177526 Free PMC article.
-
Superior colliculus resting state networks in post-traumatic stress disorder and its dissociative subtype.Hum Brain Mapp. 2018 Jan;39(1):563-574. doi: 10.1002/hbm.23865. Epub 2017 Nov 13. Hum Brain Mapp. 2018. PMID: 29134717 Free PMC article.
-
Superior Colliculus Controls the Activity of the Substantia Nigra Pars Compacta and Ventral Tegmental Area in an Asymmetrical Manner.J Neurosci. 2025 Apr 2;45(14):e1976222024. doi: 10.1523/JNEUROSCI.1976-22.2024. J Neurosci. 2025. PMID: 39819512
References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

