The influence of extracellular superoxide on iron redox chemistry and bioavailability to aquatic microorganisms
- PMID: 22514548
- PMCID: PMC3323869
- DOI: 10.3389/fmicb.2012.00124
The influence of extracellular superoxide on iron redox chemistry and bioavailability to aquatic microorganisms
Abstract
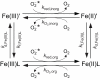
Superoxide, the one-electron reduced form of dioxygen, is produced in the extracellular milieu of aquatic microbes through a range of abiotic chemical processes and also by microbes themselves. Due to its ability to promote both oxidative and reductive reactions, superoxide may have a profound impact on the redox state of iron, potentially influencing iron solubility, complex speciation, and bioavailability. The interplay between iron, superoxide, and oxygen may also produce a cascade of other highly reactive transients in oxygenated natural waters. For microbes, the overall effect of reactions between superoxide and iron may be deleterious or beneficial, depending on the organism and its chemical environment. Here I critically discuss recent advances in understanding: (i) sources of extracellular superoxide in natural waters, with a particular emphasis on microbial generation; (ii) the chemistry of reactions between superoxide and iron; and (iii) the influence of these processes on iron bioavailability and microbial iron nutrition.
Keywords: bioavailability; iron; superoxide.
Figures


References
-
- Morel F. M. M., Hering J. G. (1993). Principles and Applications of Aquatic Chemistry. New York: Wiley
-
- Rose A. L., Waite T. D. (2002). Kinetic model for Fe(II) oxidation in seawater in the absence and presence of natural organic matter. Environ. Sci. Technol. 36, 433–444 - PubMed
-
- Afanas’ev I. B. (1989). Superoxide Ion Chemistry and Biological Implications. Boca Raton, FL: CRC Press
LinkOut - more resources
Full Text Sources

