Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition
- PMID: 22514726
- PMCID: PMC3325957
- DOI: 10.1371/journal.pone.0035312
Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition
Abstract
Background: Abdominal aortic aneurysm (AAA) is a complex multi-factorial disease with life-threatening complications. AAA is typically asymptomatic and its rupture is associated with high mortality rate. Both environmental and genetic risk factors are involved in AAA pathogenesis. Aim of this study was to investigate telomere length (TL) and oxidative DNA damage in paired blood lymphocytes, aortic endothelial cells (EC), vascular smooth muscle cells (VSMC), and epidermal cells from patients with AAA in comparison with matched controls.
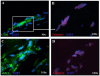
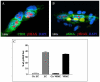
Methods: TL was assessed using a modification of quantitative (Q)-FISH in combination with immunofluorescence for CD31 or α-smooth muscle actin to detect EC and VSMC, respectively. Oxidative DNA damage was investigated by immunofluorescence staining for 7, 8-dihydro-8-oxo-2'-deoxyguanosine (8-oxo-dG).
Results and conclusions: Telomeres were found to be significantly shortened in EC, VSMC, keratinocytes and blood lymphocytes from AAA patients compared to matched controls. 8-oxo-dG immunoreactivity, indicative of oxidative DNA damage, was detected at higher levels in all of the above cell types from AAA patients compared to matched controls. Increased DNA double strand breaks were detected in AAA patients vs controls by nuclear staining for γ-H2AX histone. There was statistically significant inverse correlation between TL and accumulation of oxidative DNA damage in blood lymphocytes from AAA patients. This study shows for the first time that EC and VSMC from AAA have shortened telomeres and oxidative DNA damage. Similar findings were obtained with circulating lymphocytes and keratinocytes, indicating the systemic nature of the disease. Potential translational implications of these findings are discussed.
Conflict of interest statement
Figures





References
-
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. - PubMed
-
- Chan SW, Blackburn EH. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene. 2002;21:553–563. - PubMed
-
- Flores I, Benetti R, Blasco MA. Telomerase regulation and stem cell behavior. Curr Opin Cell Biol. 2006;18:254–260. - PubMed
-
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

