Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma
- PMID: 22516051
- PMCID: PMC3378977
- DOI: 10.1016/j.yexcr.2012.03.023
Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma
Abstract
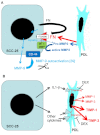
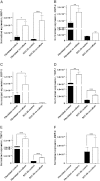
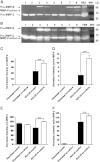
Co-culture of periodontal ligament (PDL) fibroblasts and SCC-25 oral squamous carcinoma cells (OSCC), results in conversion of PDLs into carcinoma-associated fibroblasts (CAFs). Paracrin circuits between CAFs and OSCC cells were hypothesized to regulate the gene expression of matrix remodeling enzymes in their co-culture, which was performed for 7days, followed by analysis of the mRNA/protein expression and activity of metalloproteinases (MMPs), their tissue inhibitors (TIMPs) and other relevant genes. Interleukin1-β, transforming growth factor-β1, fibronectin and αvβ6 integrin have shown to be involved in the regulation of the MMP and TIMP gene expression in co-culture of CAFs and tumor cells. In addition, these cells also cooperated in activation of MMP pro-enzymes. It is particularly interesting that the fibroblast-produced inactive MMP-2 has been activated by the tumor-cell-produced membrane-type 1 matrix metalloproteinase (MT1-MMP). The crosstalk between cancer- and the surrounding fibroblast stromal-cells is essential for the fine tuning of cancer cells invasivity.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Mamelle G., Pampurik J., Luboinski B., Lancar R., Lusinchi A., Bosq J. Lymph node prognostic factors in head and neck squamous cell carcinomas. Am. J. Surg. 1994;168:494–498. - PubMed
-
- Villaret D.B., Wang T., Dillon D., Xu J., Sivam D., Cheever M.A., Reed S.G. Identification of genes overexpressed in head and neck squamous cell carcinoma using a combination of complementary DNA subtraction and microarray analysis. Laryngoscope. 2000;110:374–381. - PubMed
-
- Ruokolainen H., Paakko P., Turpeenniemi-Hujanen T. Tissue inhibitor of matrix metalloproteinase-1 is prognostic in head and neck squamous cell carcinoma: comparison of the circulating and tissue immunoreactive protein. Clin. Cancer Res. 2005;11:3257–3264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

