The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport
- PMID: 22516235
- PMCID: PMC3589992
- DOI: 10.1016/j.nbd.2012.03.030
The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport
Abstract
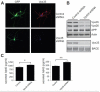
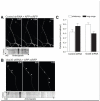
The retromer complex plays an important role in intracellular transport, is highly expressed in the hippocampus, and has been implicated in the trafficking of the amyloid precursor protein (APP). Nevertheless, the trafficking routes of the neuronal retromer and the role it plays in APP transport in neuronal processes remain unknown. Here we use hippocampal neuronal cultures to address these issues. Using fluorescence microscopy, we find that Vps35, the core element of the retromer complex, is in dendrites and axons, is enriched in endosomes and trans-Golgi network, and is found in APP-positive vesicles. Next, to identify the role the neuronal retromer plays in cargo transport, we infected hippocampal neurons with a lentivirus expressing shRNA to silence Vps35. By live fluorescence imaging, Vps35 deficiency was found to reduce the frequency, but not the kinetics, of long-range APP transport within neuronal processes. Supporting the interpretation that retromer promotes long-range transport, Vps35 deficiency led to increased APP in the early endosomes, in processes but not the soma. Finally, Vps35 deficiency was associated with increased levels of Aβ, a cleaved product of APP, increased colocalization of APP with its cleaving enzyme BACE1 in processes, and caused an enlargement of early endosomes. Taken together, our studies clarify the function of the neuronal retromer, and suggest specific mechanisms for how retromer dysfunction observed in Alzheimer's disease affects APP transport and processing.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





References
-
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–13466. - PMC - PubMed
-
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

