Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia
- PMID: 22516264
- PMCID: PMC3332001
- DOI: 10.1016/j.ccr.2012.02.018
Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia
Abstract
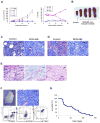
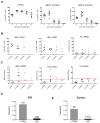
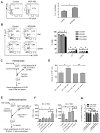
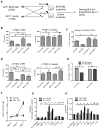
We characterized leukemia stem cells (LSC) in chronic phase chronic myelogenous leukemia (CML) using a transgenic mouse model. LSC were restricted to cells with long-term hematopoietic stem cell (LTHSC) phenotype. CML LTHSC demonstrated reduced homing and retention in the bone marrow (BM), related to decreased CXCL12 expression in CML BM, resulting from increased G-CSF production by leukemia cells. Altered cytokine expression in CML BM was associated with selective impairment of normal LTHSC growth and a growth advantage to CML LTHSC. Imatinib (IM) treatment partially corrected abnormalities in cytokine levels and LTHSC growth. These results were validated using human CML samples and provide improved understanding of microenvironmental regulation of normal and leukemic LTHSC and their response to IM in CML.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453:228–232. - PubMed
-
- Bhatia R, McGlave PB, Dewald GW, Blazar BR, Verfaillie CM. Abnormal function of the bone marrow microenvironment in chronic myelogenous leukemia: role of malignant stromal macrophages. Blood. 1995;85:3636–3645. - PubMed
-
- Cappella P, Gasparri F, Pulici M, Moll J. Cell proliferation method: click chemistry based on BrdU coupling for multiplex antibody staining. In: Paul Robinson J, editor. Current protocols in cytometry/editorial board. Unit7. Chapter 7. 2008. p. 34. - PubMed
-
- Cashman J, Clark-Lewis I, Eaves A, Eaves C. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in NOD/SCID mice. Blood. 2002;99:792–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

