Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia
- PMID: 22517358
- PMCID: PMC3404527
- DOI: 10.1152/ajpcell.00398.2011
Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia
Abstract
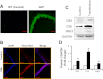
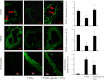
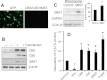
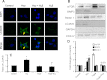
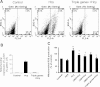
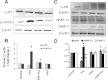
Hydrogen sulfide (H(2)S) has recently been identified as a regulator of various physiological events, including vasodilation, angiogenesis, antiapoptotic, and cellular signaling. Endogenously, H(2)S is produced as a metabolite of homocysteine (Hcy) by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3MST). Although Hcy is recognized as vascular risk factor at an elevated level [hyperhomocysteinemia (HHcy)] and contributes to vascular injury leading to renovascular dysfunction, the exact mechanism is unclear. The goal of the current study was to investigate whether conversion of Hcy to H(2)S improves renovascular function. Ex vivo renal artery culture with CBS, CSE, and 3MST triple gene therapy generated more H(2)S in the presence of Hcy, and these arteries were more responsive to endothelial-dependent vasodilation compared with nontransfected arteries treated with high Hcy. Cross section of triple gene-delivered renal arteries immunostaining suggested increased expression of CD31 and VEGF and diminished expression of the antiangiogenic factor endostatin. In vitro endothelial cell culture demonstrated increased mitophagy during high levels of Hcy and was mitigated by triple gene delivery. Also, dephosphorylated Akt and phosphorylated FoxO3 in HHcy were reversed by H(2)S or triple gene delivery. Upregulated matrix metalloproteinases-13 and downregulated tissue inhibitor of metalloproteinase-1 in HHcy were normalized by overexpression of triple genes. Together, these results suggest that H(2)S plays a key role in renovasculopathy during HHcy and is mediated through Akt/FoxO3 pathways. We conclude that conversion of Hcy to H(2)S by CBS, CSE, or 3MST triple gene therapy improves renovascular function in HHcy.
Figures

References
-
- Ahmed AK, Haylor JL, El Nahas AM, Johnson TS. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int 71: 755–763, 2007 - PubMed
-
- Border WA, Noble NA. TGF-beta in kidney fibrosis: a target for gene therapy. Kidney Int 51: 1388–1396, 1997 - PubMed
-
- Chambers JC, McGregor A, Jean-Marie J, Obeid OA, Kooner JS. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: an effect reversible with vitamin C therapy. Circulation 99: 1156–1160, 1999 - PubMed
-
- Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids 34: 573–585, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

