kNOXing on the door of selective insulin resistance
- PMID: 22517360
- PMCID: PMC3996730
- DOI: 10.1161/ATVBAHA.112.246868
kNOXing on the door of selective insulin resistance
Figures
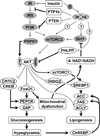
Comment on
-
NOX4 pathway as a source of selective insulin resistance and responsiveness.Arterioscler Thromb Vasc Biol. 2012 May;32(5):1236-45. doi: 10.1161/ATVBAHA.111.244525. Epub 2012 Feb 9. Arterioscler Thromb Vasc Biol. 2012. PMID: 22328777 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

