Reduction of inflammatory bowel disease-induced tumor development in IL-10 knockout mice with soluble epoxide hydrolase gene deficiency
- PMID: 22517541
- PMCID: PMC3407328
- DOI: 10.1002/mc.21918
Reduction of inflammatory bowel disease-induced tumor development in IL-10 knockout mice with soluble epoxide hydrolase gene deficiency
Abstract
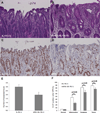
Soluble epoxide hydrolase (sEH) quickly inactivates anti-inflammatory epoxyeicosatrienoic acids (EETs) by converting them to dihydroxyeicosatrienoic acids (DHETs). Inhibition of sEH has shown effects against inflammation, but little is studied about the role of sEH in inflammatory bowel disease (IBD) and its induced carcinogenesis. In the present study, the effect of sEH gene deficiency on the development of IBD-induced tumor development was determined in IL-10 knockout mice combined with sEH gene deficiency. Tumor development in the bowel was examined at the age of 25 wk for male mice and 35 wk for female mice. Compared to IL-10(-/-) mice, sEH (-/-)/IL-10(-/-) mice exhibited a significant decrease of tumor multiplicity (2 ± 0.9 tumors/mouse vs. 1 ± 0.3 tumors/mouse) and tumor size (344.55 ± 71.73 mm³ vs. 126.94 ± 23.18 mm³), as well as a marked decrease of precancerous dysplasia. The significantly lower inflammatory scores were further observed in the bowel in sEH(-/-)/IL-10(-/-) mice as compared to IL-10(-/-) mice, including parameters of inflammation-involved area (0.70 ± 0.16 vs. 1.4 ± 0.18), inflammation cell infiltration (1.55 ± 0.35 vs. 2.15 ± 0.18), and epithelial hyperplasia (0.95 ± 0.21 vs. 1.45 ± 0.18), as well as larger ulcer formation. qPCR and Western blotting assays demonstrated a significant downregulation of cytokines/chemokines (TNF-α, MCP-1, and IL-12, 17, and 23) and NF-κB signals. Eicosanoid acid metabolic profiling revealed a significant increase of ratios of EETs to DHETs and EpOMEs to DiOMEs. These results indicate that sEH plays an important role in IBD and its-induced carcinogenesis and could serve as a highly potential target of chemoprevention and treatment for IBD.
Keywords: IL-10; carcinogenesis; eicosanoid acid metabolic profiling; inflammatory bowel disease; soluble epoxide hydrolase.
Copyright © 2012 Wiley Periodicals, Inc.
Figures



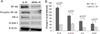


References
-
- Yang GY, Taboada S, Liao J. Inflammatory bowel disease: a model of chronic inflammation-induced cancer. Methods Mol Biol. 2009;511:193–233. - PubMed
-
- Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50(3):225–237. - PubMed
-
- Huang H, Morisseau C, Wang J, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol Renal Physiol. 2007;293(1):F342–F349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

