Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte-embryo interface
- PMID: 22517738
- PMCID: PMC3356617
- DOI: 10.1073/pnas.1119212109
Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte-embryo interface
Abstract
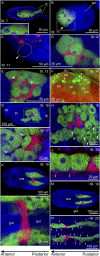
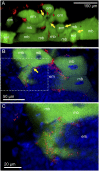
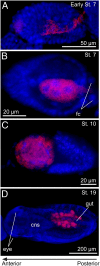
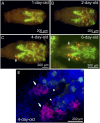
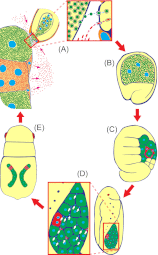
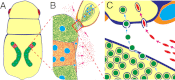
Many insects are associated with obligate symbiotic bacteria, which are localized in specialized cells called bacteriocytes, vertically transmitted through host generations via ovarial passage, and essential for growth and reproduction of their hosts. Although vertical transmission is pivotal for maintenance of such intimate host-symbiont associations, molecular and cellular mechanisms underlying the process are largely unknown. Here we report a cellular mechanism for vertical transmission of the obligate symbiont Buchnera in the pea aphid Acyrthosiphon pisum. In the aphid body, Buchnera cells are transmitted from maternal bacteriocytes to adjacent blastulae at the ovariole tips in a highly coordinated manner. By making use of symbiont-manipulated strains of A. pisum, we demonstrated that the facultative symbiont Serratia is, unlike Buchnera, not transmitted from maternal bacteriocytes to blastulae, suggesting a specific mechanism for Buchnera transmission. EM observations revealed a series of exo-/endocytotic processes operating at the bacteriocyte-blastula interface: Buchnera cells are exocytosed from the maternal bacteriocyte, temporarily released to the extracellular space, and endocytosed by the posterior syncytial cytoplasm of the blastula. These results suggest that the selective Buchnera transmission is likely attributable to Buchnera-specific exocytosis by the maternal bacteriocyte, whereas both Buchnera and Serratia are nonselectively incorporated by the endocytotic activity of the posterior region of the blastula. The sophisticated cellular mechanism for vertical transmission of Buchnera must have evolved to ensure the obligate host-symbiont association, whereas facultative symbionts like Serratia may coopt the endocytotic component of the mechanism for their entry into the host embryos.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Buchner P. Endosymbiosis of Animals with Plant Microorganisms. New York: Interscience; 1965.
-
- Werren JH, Baldo L, Clark ME. Wolbachia: Master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. - PubMed
-
- Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol. 2010;55:247–266. - PubMed
-
- Baumann P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol. 2005;59:155–189. - PubMed
-
- Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

