Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation
- PMID: 22517894
- PMCID: PMC3383012
- DOI: 10.1182/blood-2012-03-306605
Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation
Abstract
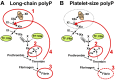
Inorganic polyphosphate is widespread in biology and exhibits striking prohemostatic, prothrombotic, and proinflammatory effects in vivo. Long-chain polyphosphate (of the size present in infectious microorganisms) is a potent, natural pathophysiologic activator of the contact pathway of blood clotting. Medium-chain polyphosphate (of the size secreted from activated human platelets) accelerates factor V activation, completely abrogates the anticoagulant function of tissue factor pathway inhibitor, enhances fibrin clot structure, and greatly accelerates factor XI activation by thrombin. Polyphosphate may have utility as a hemostatic agent, whereas antagonists of polyphosphate may function as novel antithrombotic/anti-inflammatory agents. The detailed molecular mechanisms by which polyphosphate modulates blood clotting reactions remain to be elucidated.
Figures
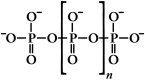

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

