The effectiveness of SPARX, a computerised self help intervention for adolescents seeking help for depression: randomised controlled non-inferiority trial
- PMID: 22517917
- PMCID: PMC3330131
- DOI: 10.1136/bmj.e2598
The effectiveness of SPARX, a computerised self help intervention for adolescents seeking help for depression: randomised controlled non-inferiority trial
Abstract
Objective: To evaluate whether a new computerised cognitive behavioural therapy intervention (SPARX, Smart, Positive, Active, Realistic, X-factor thoughts) could reduce depressive symptoms in help seeking adolescents as much or more than treatment as usual.
Design: Multicentre randomised controlled non-inferiority trial.
Setting: 24 primary healthcare sites in New Zealand (youth clinics, general practices, and school based counselling services).
Participants: 187 adolescents aged 12-19, seeking help for depressive symptoms, with no major risk of self harm and deemed in need of treatment by their primary healthcare clinicians: 94 were allocated to SPARX and 93 to treatment as usual.
Interventions: Computerised cognitive behavioural therapy (SPARX) comprising seven modules delivered over a period of between four and seven weeks, versus treatment as usual comprising primarily face to face counselling delivered by trained counsellors and clinical psychologists.
Outcomes: The primary outcome was the change in score on the children's depression rating scale-revised. Secondary outcomes included response and remission on the children's depression rating scale-revised, change scores on the Reynolds adolescent depression scale-second edition, the mood and feelings questionnaire, the Kazdin hopelessness scale for children, the Spence children's anxiety scale, the paediatric quality of life enjoyment and satisfaction questionnaire, and overall satisfaction with treatment ratings.
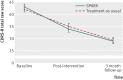
Results: 94 participants were allocated to SPARX (mean age 15.6 years, 62.8% female) and 93 to treatment as usual (mean age 15.6 years, 68.8% female). 170 adolescents (91%, SPARX n = 85, treatment as usual n = 85) were assessed after intervention and 168 (90%, SPARX n = 83, treatment as usual n = 85) were assessed at the three month follow-up point. Per protocol analyses (n = 143) showed that SPARX was not inferior to treatment as usual. Post-intervention, there was a mean reduction of 10.32 in SPARX and 7.59 in treatment as usual in raw scores on the children's depression rating scale-revised (between group difference 2.73, 95% confidence interval -0.31 to 5.77; P=0.079). Remission rates were significantly higher in the SPARX arm (n = 31, 43.7%) than in the treatment as usual arm (n = 19, 26.4%) (difference 17.3%, 95% confidence interval 1.6% to 31.8%; P = 0.030) and response rates did not differ significantly between the SPARX arm (66.2%, n = 47) and treatment as usual arm (58.3%, n = 42) (difference 7.9%, -7.9% to 24%; P = 0.332). All secondary measures supported non-inferiority. Intention to treat analyses confirmed these findings. Improvements were maintained at follow-up. The frequency of adverse events classified as "possibly" or "probably" related to the intervention did not differ between groups (SPARX n = 11; treatment as usual n = 11).
Conclusions: SPARX is a potential alternative to usual care for adolescents presenting with depressive symptoms in primary care settings and could be used to address some of the unmet demand for treatment.
Trial registration: Australian New Zealand Clinical Trials ACTRN12609000249257.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
SPARX computerised CBT is as effective as usual care for mild-to-moderate depression in help seeking adolescents.Evid Based Ment Health. 2012 Nov;15(4):90. doi: 10.1136/ebmental-2012-100822. Epub 2012 Jul 7. Evid Based Ment Health. 2012. PMID: 22773784 No abstract available.
-
A computerized self-help intervention is as effective as face-to-face counseling for adolescents seeking help for depression.J Pediatr. 2012 Nov;161(5):967-8. doi: 10.1016/j.jpeds.2012.08.050. J Pediatr. 2012. PMID: 23095695 No abstract available.
References
-
- Lewinsohn PM, Rohde P, Seeley JR. Major depressive disorder in older adolescents: prevalence, risk factors and clinical implications. Clin Psychol Rev 1998;18:765-94. - PubMed
-
- World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. WHO, 2009.
-
- Watanabe N, Hunot V, Omori IM, Churchill R, Furukawa TA. Psychotherapy for depression among children and adolescents: a systematic review. Acta Psychiatr Scand 2007;116:84-95. - PubMed
-
- National Institute for Health and Clinical Excellence. Depression in children and young people: identification and management in primary, community and secondary care. NICE, 2005. - PubMed
-
- Kataoka S, Zhang L, Wells K. Unmet need for mental health care among U.S. children: variations by ethnicity and insurance status. Am J Psychiatry 2002;159:1548-55. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical