Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play
- PMID: 22517954
- PMCID: PMC3329271
- DOI: 10.1148/radiol.12110433
Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play
Abstract
Alzheimer disease (AD) is one of, if not the most, feared diseases associated with aging. The prevalence of AD increases exponentially with age after 60 years. Increasing life expectancy coupled with the absence of any approved disease-modifying therapies at present position AD as a dominant public health problem. Major advances have occurred in the development of disease biomarkers for AD in the past 2 decades. At present, the most well-developed AD biomarkers are the cerebrospinal fluid analytes amyloid-β 42 and tau and the brain imaging measures amyloid positron emission tomography (PET), fluorodeoxyglucose PET, and magnetic resonance imaging. CSF and imaging biomarkers are incorporated into revised diagnostic guidelines for AD, which have recently been updated for the first time since their original formulation in 1984. Results of recent studies suggest the possibility of an ordered evolution of AD biomarker abnormalities that can be used to stage the typical 20-30-year course of the disease. When compared with biomarkers in other areas of medicine, however, the absence of standardized quantitative metrics for AD imaging biomarkers constitutes a major deficiency. Failure to move toward a standardized system of quantitative metrics has substantially limited potential diagnostic usefulness of imaging in AD. This presents an important opportunity that, if widely embraced, could greatly expand the application of imaging to improve clinical diagnosis and the quality and efficiency of clinical trials.
Figures



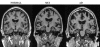



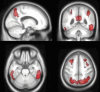
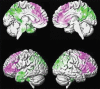
References
-
- Alzheimer A. Uber eigenartige Krankheitsfalle des spateren Alters. Z Gesamte Neurol Psychiatr 1911;4(1):356–385
-
- Alzheimer’s Association 2010 Alzheimer’s disease facts and figures. Alzheimers Dement 2010;6(2):158–194 - PubMed
-
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep 2009;57(14):1–134 - PubMed
-
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58(12):1985–1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

