Liver angiotensinogen is the primary source of renal angiotensin II
- PMID: 22518004
- PMCID: PMC3380650
- DOI: 10.1681/ASN.2011121159
Liver angiotensinogen is the primary source of renal angiotensin II
Abstract
Angiotensin II content in the kidney is much higher than in the plasma, and it increases more in kidney diseases through an uncertain mechanism. Because the kidney abundantly expresses angiotensinogen mRNA, transcriptional dysregulation of angiotensinogen within the kidney is one potential cause of increased renal angiotensin II in the setting of disease. Here, we observed that kidney-specific angiotensinogen knockout mice had levels of renal angiotensinogen protein and angiotensin II that were similar to those levels of control mice. In contrast, liver-specific knockout of angiotensinogen nearly abolished plasma and renal angiotensinogen protein and renal tissue angiotensin II. Immunohistochemical analysis in mosaic proximal tubules of megalin knockout mice revealed that angiotensinogen protein was incorporated selectively in megalin-intact cells of the proximal tubule, indicating that the proximal tubule reabsorbs filtered angiotensinogen through megalin. Disruption of the filtration barrier in a transgenic mouse model of podocyte-selective injury increased renal angiotensin II content and markedly increased both tubular and urinary angiotensinogen protein without an increase in renal renin activity, supporting the dependency of renal angiotensin II generation on filtered angiotensinogen. Taken together, these data suggest that liver-derived angiotensinogen is the primary source of renal angiotensinogen protein and angiotensin II. Furthermore, an abnormal increase in the permeability of the glomerular capillary wall to angiotensinogen, which characterizes proteinuric kidney diseases, enhances the synthesis of renal angiotensin II.
Figures
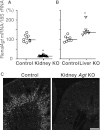



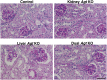
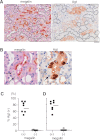
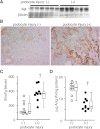
Comment in
-
The increasing complexity of the intratubular Renin-Angiotensin system.J Am Soc Nephrol. 2012 Jul;23(7):1130-2. doi: 10.1681/ASN.2012050493. Epub 2012 Jun 7. J Am Soc Nephrol. 2012. PMID: 22677556 No abstract available.
References
-
- Navar LG, Nishiyama A: Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens 13: 107–115, 2004 - PubMed
-
- Kobori H, Nangaku M, Navar LG, Nishiyama A: The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 - PubMed
-
- Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC: Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006 - PubMed
-
- Bader M, Ganten D: Update on tissue renin-angiotensin systems. J Mol Med (Berl) 86: 615–621, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

