Synergistic Catalysis: A Powerful Synthetic Strategy for New Reaction Development
- PMID: 22518271
- PMCID: PMC3327486
- DOI: 10.1039/C2SC00907B
Synergistic Catalysis: A Powerful Synthetic Strategy for New Reaction Development
Abstract
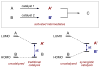
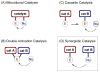
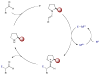
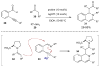
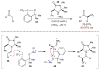
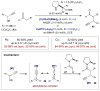
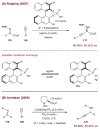
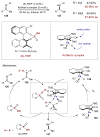
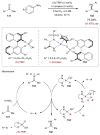
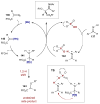
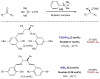
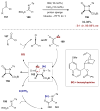
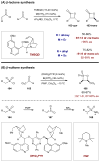
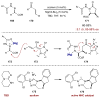
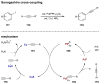
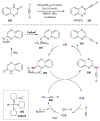
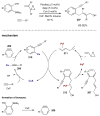
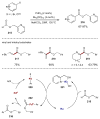
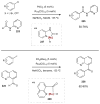
Synergistic catalysis is a synthetic strategy wherein both the nucleophile and the electrophile are simultaneously activated by two separate and distinct catalysts to afford a single chemical transformation. This powerful catalysis strategy leads to several benefits, specifically synergistic catalysis can (i) introduce new, previously unattainable chemical transformations, (ii) improve the efficiency of existing transformations, and (iii) create or improve catalytic enantioselectivity where stereocontrol was previously absent or challenging. This perspective aims to highlight these benefits using many of the successful examples of synergistic catalysis found in the literature.
Figures



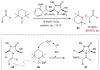
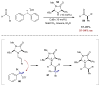

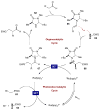
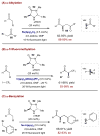











References
-
-
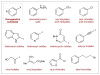
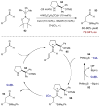
For reviews on bifunctional catalysis, see: Shibasaki M, Kanai M, Matsunaga S, Kumagai N. Acc Chem Res. 2009;42:1117.Breslow R. J Molec Catal. 1994;91:161.Shibasaki M, Yoshikawa N. Chem Rev. 2002;102:2187.Wang Y, Deng L. In: Catalytic Asymmetric Synthesis. 3. Ojima I, editor. John Wiley & Sons; New Jersey: 2010. p. 59.Shibasaki M, Kanai M. In: New Frontiers in Asymmetric Catalysis. Mikami K, Lautens M, editors. John Wiley & Sons; New Jersey: 2007. p. 383.
-
-
-
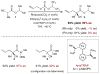
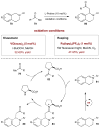
For examples of double activation catalysis, see: Xu H, Zuend SJ, Woll MG, Tao Y, Jacobsen EN. Science. 2010;327:986.Rubina M, Conley M, Gevorgyan V. J Am Chem Soc. 2006;128:5818.Mukherjee S, List B. J Am Chem Soc. 2007;129:11336.Shi Y, Peterson SM, Haberaecker WW, III, Blum SA. J Am Chem Soc. 2008;130:2168.Park YJ, Park JW, Jun CH. Acc Chem Res. 2008;41:222.
-
-
-
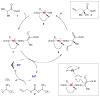
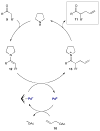
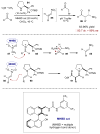
For examples of cascade catalysis, see: Belot S, Vogt KA, Besnard C, Krause N, Alexakis A. Angew Chem Int Ed. 2009;48:8923.Han ZY, Xiao H, Chen XH, Gong LZ. J Am Chem Soc. 2009;131:9182.Sorimachi K, Terada M. J Am Chem Soc. 2008;130:14452.Cai Q, Zhao ZA, You SL. Angew Chem Int Ed. 2009;48:7428.Simmons B, Walji AM, MacMillan DWC. J Am Chem Soc. 2009;48:4349.Lathrop SP, Rovis T. J Am Chem Soc. 2009;131:13628.
-
-
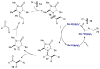
- Strater N, Lipscomb WN, Klabunde T, Krebs B. Angew Chem Int Ed. 1996;35:2024.
-
- Brown KA, Kraut J. Faraday Discuss. 1992;93:217. - PubMed
- Polshakov VI. Russ Chem Bull, Int Ed. 2001;50:1733.
Grants and funding
LinkOut - more resources
Full Text Sources

