Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention
- PMID: 22518340
- PMCID: PMC3299397
- DOI: 10.1155/2012/658786
Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention
Abstract
Chronic inflammation is a well-recognized risk factor for development of human cancer in several tissues, including large bowel. Inflammatory bowel disease, including ulcerative colitis and Crohn's disease, is a longstanding inflammatory disease of intestine with increased risk for colorectal cancer development. Several molecular events involved in chronic inflammatory process may contribute to multistep carcinogenesis of human colorectal cancer in the inflamed colon. They include overproduction of reactive oxygen and nitrogen species, overproduction and upregulation of productions and enzymes of arachidonic acid biosynthesis pathway and cytokines, and intestinal immune system dysfunction. In this paper, I will describe several methods to induce colorectal neoplasm in the inflamed colon. First, I will introduce a protocol of a novel inflammation-associated colon carcinogenesis in mice. In addition, powerful tumor-promotion/progression activity of dextran sodium sulfate in the large bowel of Apc(Min/+) mice will be described. Finally, chemoprevention of inflammation-associated colon carcinogenesis will be mentioned.
Figures


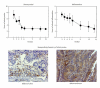
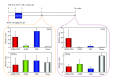
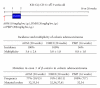

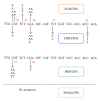



References
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? The Lancet. 2001;357(9255):539–545. - PubMed
-
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. The Lancet. 1984;1(8390):1311–1314. - PubMed
-
- Tanaka T, Kohno H, Murakami M, Shimada R, Kagami S. Colitis-related rat colon carcinogenesis induced by 1-hydroxyanthraquinone and methylazoxymethanol acetate (review) Oncology Reports. 2000;7(3):501–508. - PubMed
-
- Sung JJY, Lau JYW, Goh KL, et al. Increasing incidence of colorectal cancer in Asia: implications for screening. The Lancet Oncology. 2005;6(11):871–876. - PubMed
LinkOut - more resources
Full Text Sources

