Blockade of β-adrenoceptors restores the GRK2-mediated adrenal α(2) -adrenoceptor-catecholamine production axis in heart failure
- PMID: 22519418
- PMCID: PMC3448904
- DOI: 10.1111/j.1476-5381.2012.01972.x
Blockade of β-adrenoceptors restores the GRK2-mediated adrenal α(2) -adrenoceptor-catecholamine production axis in heart failure
Abstract
Background and purpose: Sympathetic nervous system (SNS) hyperactivity is characteristic of chronic heart failure (HF) and significantly worsens prognosis. The success of β-adrenoceptor antagonist (β-blockers) therapy in HF is primarily attributed to protection of the heart from the noxious effects of augmented catecholamine levels. β-Blockers have been shown to reduce SNS hyperactivity in HF, but the underlying molecular mechanisms are not understood. The GPCR kinase-2 (GRK2)-α(2) adrenoceptor-catecholamine production axis is up-regulated in the adrenal medulla during HF causing α(2) -adrenoceptor dysfunction and elevated catecholamine levels. Here, we sought to investigate if β-blocker treatment in HF could lower SNS activation by directly altering adrenal GRK2 levels.
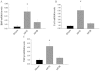
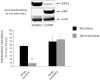
Experimental approach: Four weeks after myocardial infarction-induced HF, adult rats were randomized to 10-week treatment with vehicle (HF/C) or bisoprolol (HF/B). Cardiac function and dimensions were measured. In heart and adrenal gland, GRK2 levels were assessed by RT-PCR and Western blotting and adrenoceptors studied with radioligand binding. Catecholamines and α(2) adrenoceptors in adrenal medulla chromaffin cell cultures were also measured.
Key results: Bisoprolol treatment ameliorated HF-related adverse cardiac remodelling and reduced plasma catecholamine levels, compared with HF/C rats. Bisoprolol also attenuated adrenal GRK2 overexpression as observed in HF/C rats and increased α(2) adrenoceptor density. In cultures of adrenal medulla chromaffin cells from all study groups, bisoprolol reversed HF-related α(2) adrenoceptor dysfunction. This effect was reversed by GRK2 overexpression.
Conclusion and implications: Blockade of β-adrenoceptors normalized the adrenal α(2) adrenoceptor-catecholamine production axis by reducing GRK2 levels. This effect may contribute significantly to the decrease of HF-related sympathetic overdrive by β-blockers.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures
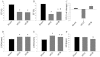



References
-
- Al-Hesayen A, Azevedo ER, Floras JS, Hollingshead S, Lopaschuk GD, Parker JD. Selective versus nonselective beta-adrenergic receptor blockade in chronic heart failure: differential effects on myocardial energy substrate utilization. Eur J Heart Fail. 2005;7:618–623. - PubMed
-
- Andersson B, Hamm C, Persson S, Wikström G, Sinagra G, Hjalmarson A, et al. Improved exercise hemodynamic status in dilated cardiomyopathy after beta-adrenergic blockade treatment. J Am Coll Cardiol. 1994;23:1397–1404. - PubMed
-
- Blanchet M, Ducharme A, Racine N, Rouleau JL, Tardif JC, Juneau M, et al. Effects of cold exposure on submaximal exercise performance and adrenergic activation in patients with congestive heart failure and the effects of beta-adrenergic blockade (carvedilol or metoprolol) Am J Cardiol. 2003;92:548–553. - PubMed
-
- Bristow MR. Mechanistic and clinical rationales for using beta-blockers in heart failure. J Card Fail. 2000a;6:8–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

