Protein misfolding and aggregation in cataract disease and prospects for prevention
- PMID: 22520268
- PMCID: PMC3621977
- DOI: 10.1016/j.molmed.2012.03.005
Protein misfolding and aggregation in cataract disease and prospects for prevention
Abstract
The transparency of the eye lens depends on maintaining the native tertiary structures and solubility of the lens crystallin proteins over a lifetime. Cataract, the leading cause of blindness worldwide, is caused by protein aggregation within the protected lens environment. With age, covalent protein damage accumulates through pathways thought to include UV radiation, oxidation, deamidation, and truncations. Experiments suggest that the resulting protein destabilization leads to partially unfolded, aggregation-prone intermediates and the formation of insoluble, light-scattering protein aggregates. These aggregates either include or overwhelm the protein chaperone content of the lens. Here, we review the causes of cataract and nonsurgical methods being investigated to inhibit or delay cataract development, including natural product-based therapies, modulators of oxidation, and protein aggregation inhibitors.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
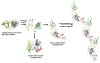


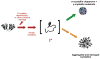
References
-
- Michael R, et al. Changes in the refractive index of lens fibre membranes during maturation--impact on lens transparency. Exp Eye Res. 2003;77:93–99. - PubMed
-
- Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. - PubMed
-
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. - PubMed
-
- Oyster CW. The human eye: structure and function. Sinauer Associates, Inc; 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

