The changing limits and incidence of malaria in Africa: 1939-2009
- PMID: 22520443
- PMCID: PMC3521063
- DOI: 10.1016/B978-0-12-394303-3.00010-4
The changing limits and incidence of malaria in Africa: 1939-2009
Abstract
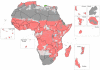
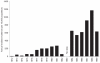
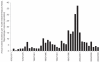
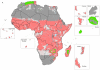
Understanding the historical, temporal changes of malaria risk following control efforts in Africa provides a unique insight into what has been and might be archived towards a long-term ambition of elimination on the continent. Here, we use archived published and unpublished material combined with biological constraints on transmission accompanied by a narrative on malaria control to document the changing incidence of malaria in Africa since earliest reports pre-second World War. One result is a more informed mapped definition of the changing margins of transmission in 1939, 1959, 1979, 1999 and 2009.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

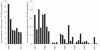
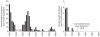


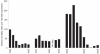
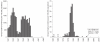

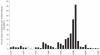






References
-
- Afripop Project Map of population density Africa. 2011 http://www.afripop.org.
-
- Albonico M, Giorgi FD, Razanakolona J, Raveloson A, Sabatinelli G, Pietra V, et al. Control of epidemic malaria on the highlands of Madagascar. Parassitologia. 1999;41:373–376. - PubMed
-
- Ali Halidi ME. Paludisme à Mayotte: passé, présent, futur. Sante. 1995;5:362–367. - PubMed
-
- Alves W. Preliminary notes on a southern Rhodesian experiment in malaria control. S. Afr. J. Sci. 1951;47:289–292.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

