The antifolates
- PMID: 22520983
- PMCID: PMC3777421
- DOI: 10.1016/j.hoc.2012.02.002
The antifolates
Abstract
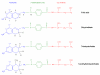
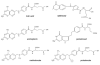
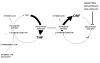
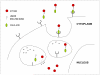
This article focuses on the cellular, biochemical, and molecular pharmacology of antifolates and how a basic understanding of the mechanism of action of methotrexate, its cytotoxic determinants, mechanisms of resistance, and transport into and out of cells has led to the development of a new generation of antifolates, a process that continues in the laboratory and in the clinics. New approaches to folate-based cancer chemotherapy are described based on the targeted delivery of drugs to malignant cells.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Skipper HE, Perry S. Kinetics of normal and leukemic leukocyte populations and relevance to chemotherapy. Cancer Res. 1970;30:1883–97. - PubMed
-
- Goldin A, Venditti JM, Humphreys SR, et al. A quantitative comparison of the antileukemic effectiveness of two folic acid antagonists in mice. J Natl Cancer Inst. 1955;15:1657–64. - PubMed
-
- Farber S, Diamond LK, Mercer RD, et al. Temporary remission in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl glutamic acid (aminopterin) N Engl J Med. 1948;238:787–93. - PubMed
-
- Goldman ID, Chattopadhyay S, Zhao R, et al. The Antifolates: Evolution, New Agents in the Clinic, and How targeting delivery via specific membrane transporters is driving the development of a next generation of folate analogs. Curr Opin Investig Drugs. 2010;11:1409–23. - PubMed
-
- Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

