Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab
- PMID: 22522603
- PMCID: PMC3410971
- DOI: 10.1002/pbc.24188
Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab
Abstract
Background: Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome that remains difficult to treat. Even with current standard HLH therapy, only approximately half of patients will experience complete resolution of disease, and early mortality remains a significant problem. Salvage therapies have been described only in limited case reports, and there are no large studies of second-line therapies.
Procedure: We reviewed the charts of 22 pediatric and adult patients who received alemtuzumab for the treatment of refractory HLH at our center or in consultation with our group.
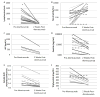
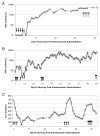
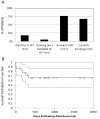
Results: Patients had received conventional therapies for a median of 8 weeks (range: 2-70) prior to alemtuzumab, and treatment immediately prior to alemtuzumab included dexamethasone (100%), etoposide (77%), cyclosporine (36%), intrathecal hydrocortisone ± methotrexate (23%), methylprednisolone (9%), and rituximab (14%). Patients received a median dose of 1 mg/kg alemtuzumab (range: 0.1-8.9 mg/kg) divided over a median of 4 days (range: 2-10). Fourteen patients experienced an overall partial response, defined as at least a 25% improvement in two or more quantifiable symptoms or laboratory markers of HLH 2 weeks following alemtuzumab (64%). Five additional patients had a 25% or greater improvement in a single quantifiable symptom or laboratory marker of HLH (23%). Seventy-seven percent of patients survived to undergo allogeneic hematopoietic cell transplantation. Patients experienced an acceptable spectrum of complications, including CMV and adenovirus viremia.
Conclusion: Alemtuzumab appears to be an effective salvage agent for refractory HLH, leading to improvement and survival to HCT in many patients. Prospective trials to define optimal dosing levels, schedules, and responses are needed.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
-
- Henter JI, Samuelsson-Horne A, Arico M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367–2373. - PubMed
-
- Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. - PubMed
-
- Mahlaoui N, Ouachee-Chardin M, de Saint Basile G, et al. Immunotherapy of familial hemophagocytic lymphohistiocytosis with antithymocyte globulins: a single-center retrospective report of 38 patients. Pediatrics. 2007;120(3):e622–628. - PubMed
-
- Baker KS, Filipovich AH, Gross TG, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2008;42(3):175–180. - PubMed
-
- Horne A, Janka G, Maarten Egeler R, et al. Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis. Br J Haematol. 2005;129(5):622–630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources