Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance
- PMID: 22522611
- PMCID: PMC3379662
- DOI: 10.2337/db11-0832
Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance
Abstract
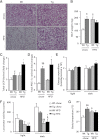
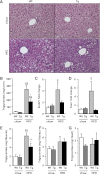
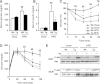
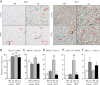
During the expansion of fat mass in obesity, vascularization of adipose tissue is insufficient to maintain tissue normoxia. Local hypoxia develops and may result in altered adipokine expression, proinflammatory macrophage recruitment, and insulin resistance. We investigated whether an increase in adipose tissue angiogenesis could protect against obesity-induced hypoxia and, consequently, insulin resistance. Transgenic mice overexpressing vascular endothelial growth factor (VEGF) in brown adipose tissue (BAT) and white adipose tissue (WAT) were generated. Vessel formation, metabolism, and inflammation were studied in VEGF transgenic mice and wild-type littermates fed chow or a high-fat diet. Overexpression of VEGF resulted in increased blood vessel number and size in both WAT and BAT and protection against high-fat diet-induced hypoxia and obesity, with no differences in food intake. This was associated with increased thermogenesis and energy expenditure. Moreover, whole-body insulin sensitivity and glucose tolerance were improved. Transgenic mice presented increased macrophage infiltration, with a higher number of M2 anti-inflammatory and fewer M1 proinflammatory macrophages than wild-type littermates, thus maintaining an anti-inflammatory milieu that could avoid insulin resistance. These studies suggest that overexpression of VEGF in adipose tissue is a potential therapeutic strategy for the prevention of obesity and insulin resistance.
Figures




Comment in
-
Comment on: Elias et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 2012;61:1801-1813.Diabetes. 2013 Jan;62(1):e3. doi: 10.2337/db12-1130. Diabetes. 2013. PMID: 23258919 Free PMC article. No abstract available.
References
-
- James WP. The epidemiology of obesity: the size of the problem. J Intern Med 2008;263:336–352 - PubMed
-
- Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007;56:901–911 - PubMed
-
- Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

