Circulating preproinsulin signal peptide-specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill β-cells
- PMID: 22522618
- PMCID: PMC3379678
- DOI: 10.2337/db11-1520
Circulating preproinsulin signal peptide-specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill β-cells
Abstract
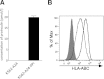
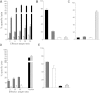
Type 1 diabetes results from T cell-mediated β-cell destruction. The HLA-A*24 class I gene confers significant risk of disease and early onset. We tested the hypothesis that HLA-A24 molecules on islet cells present preproinsulin (PPI) peptide epitopes to CD8 cytotoxic T cells (CTLs). Surrogate β-cell lines secreting proinsulin and expressing HLA-A24 were generated and their peptide ligandome examined by mass spectrometry to discover naturally processed and HLA-A24-presented PPI epitopes. A novel PPI epitope was identified and used to generate HLA-A24 tetramers and examine the frequency of PPI-specific T cells in new-onset HLA-A*24(+) patients and control subjects. We identified a novel naturally processed and HLA-A24-presented PPI signal peptide epitope (PPI(3-11); LWMRLLPLL). HLA-A24 tetramer analysis reveals a significant expansion of PPI(3-11)-specific CD8 T cells in the blood of HLA-A*24(+) recent-onset patients compared with HLA-matched control subjects. Moreover, a patient-derived PPI(3-11)-specific CD8 T-cell clone shows a proinflammatory phenotype and kills surrogate β-cells and human HLA-A*24(+) islet cells in vitro. These results indicate that the type 1 diabetes susceptibility molecule HLA-A24 presents a naturally processed PPI signal peptide epitope. PPI-specific, HLA-A24-restricted CD8 T cells are expanded in patients with recent-onset disease. Human islet cells process and present PPI(3-11), rendering themselves targets for CTL-mediated killing.
Figures





References
-
- Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 1985;313:353–360 - PubMed
-
- Foulis AK, Farquharson MA, Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1987;30:333–343 - PMC - PubMed
-
- Itoh N, Hanafusa T, Miyazaki A, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest 1993;92:2313–2322 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

