Systematic mapping of fragile X granules in the mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions
- PMID: 22522693
- PMCID: PMC3998511
- DOI: 10.1002/cne.23123
Systematic mapping of fragile X granules in the mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions
Abstract
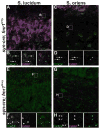
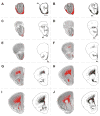
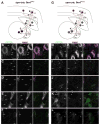
Loss of Fragile X mental retardation protein (FMRP) leads to Fragile X syndrome (FXS), the most common form of inherited intellectual disability and autism. Although the functions of FMRP and its homologs FXR1P and FXR2P are well studied in the somatodendritic domain, recent evidence suggests that this family of RNA binding proteins also plays a role in the axonal and presynaptic compartments. Fragile X granules (FXGs) are morphologically and genetically defined structures containing Fragile X proteins that are expressed axonally and presynaptically in a subset of circuits. To further understand the role of presynaptic Fragile X proteins in the brain, we systematically mapped the FXG distribution in the mouse central nervous system. This analysis revealed both the circuits and the neuronal types that express FXGs. FXGs are enriched in circuits that mediate sensory processing and motor planning-functions that are particularly perturbed in FXS patients. Analysis of FXG expression in the hippocampus suggests that CA3 pyramidal neurons use presynaptic Fragile X proteins to modulate recurrent but not feedforward processing. Neuron-specific FMRP mutants revealed a requirement for neuronal FMRP in the regulation of FXGs. Finally, conditional FMRP ablation demonstrated that FXGs are expressed in axons of thalamic relay nuclei that innervate cortex, but not in axons of thalamic reticular nuclei, striatal nuclei, or cortical neurons that innervate thalamus. Together, these findings support the proposal that dysregulation of axonal and presynaptic Fragile X proteins contribute to the neurological symptoms of FXS.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest: The authors declare no competing interests.
Figures








References
-
- Baranek GT, Roberts JE, David FJ, Sideris J, Mirrett PL, Hatton DD, Bailey DB., Jr Developmental trajectories and correlates of sensory processing in young boys with fragile X syndrome. Phys Occup Ther Pediatr. 2008;28:79–98. - PubMed
-
- Bontekoe CJM, McIlwain KL, Nieuwenhuizen IM, Yuva-Paylor LA, Nellis A, Willemsen R, Fang Z, Kirkpatrick L, Bakker CE, McAninch R, et al. Knockout mouse model for Fxr2: a model for mental retardation. Hum Mol Genet. 2002;11:487–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

