Macroautophagy is deregulated in murine and human lupus T lymphocytes
- PMID: 22522825
- PMCID: PMC3429547
- DOI: 10.4161/auto.20275
Macroautophagy is deregulated in murine and human lupus T lymphocytes
Abstract
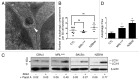
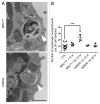
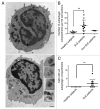
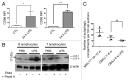
Macroautophagy was recently shown to regulate both lymphocyte biology and innate immunity. In this study we sought to determine whether a deregulation of autophagy was linked to the development of autoimmunity. Genome-wide association studies have pointed out nucleotide polymorphisms that can be associated with systemic lupus erythematosus, but the potential role of autophagy in the initiation and/or development of this syndrome is still unknown. Here, we provide first clues of macroautophagy deregulation in lupus. By the use of LC3 conversion assays and electron microscopy experiments, we observed that T cells from two distinct lupus-prone mouse models, i.e., MRL (lpr/lpr) and (NZB/NZW)F1, exhibit high loads of autophagic compartments compared with nonpathologic control CBA/J and BALB/c mice. Unlike normal mice, autophagy increases with age in murine lupus. In vivo lipopolysaccharide stimulation in CBA/J control mice efficiently activates T lymphocytes but fails to upregulate formation of autophagic compartments in these cells. This argues against a deregulation of autophagy in lupus T cells solely resulting from an acute inflammation injury. Autophagic vacuoles quantified by electron microscopy are also found to be significantly more frequent in T cells from lupus patients compared with healthy controls and patients with non-lupus autoimmune diseases. This elevated number of autophagic structures is not distributed homogeneously and appears to be more pronounced in certain T cells. These results suggest that autophagy could regulate the survival of autoreactive T cell during lupus, and could thus lead to design new therapeutic options for lupus.
Keywords: T lymphocytes; lupus-prone mice; macroautophagy; systemic lupus erythematosus.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
