In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes
- PMID: 22522929
- PMCID: PMC3369107
- DOI: 10.1038/nature11044
In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes
Abstract
The reprogramming of adult cells into pluripotent cells or directly into alternative adult cell types holds great promise for regenerative medicine. We reported previously that cardiac fibroblasts,which represent 50%of the cells in the mammalian heart, can be directly reprogrammed to adult cardiomyocyte-like cells in vitro by the addition of Gata4, Mef2c and Tbx5 (GMT). Here we use genetic lineage tracing to show that resident non-myocytes in the murine heart can be reprogrammed into cardiomyocyte-like cells in vivo by local delivery of GMT after coronary ligation. Induced cardiomyocytes became binucleate, assembled sarcomeres and had cardiomyocyte-like gene expression. Analysis of single cells revealed ventricular cardiomyocyte-like action potentials, beating upon electrical stimulation, and evidence of electrical coupling. In vivo delivery of GMT decreased infarct size and modestly attenuated cardiac dysfunction up to 3 months after coronary ligation. Delivery of the pro-angiogenic and fibroblast-activating peptide, thymosin b4, along with GMT, resulted in further improvements in scar area and cardiac function. These findings demonstrate that cardiac fibroblasts can be reprogrammed into cardiomyocyte-like cells in their native environment for potential regenerative purposes.
Figures
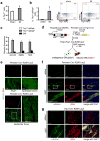
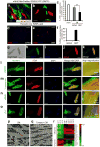


Comment in
-
Regenerative medicine: Reprogramming the injured heart.Nature. 2012 May 31;485(7400):585-6. doi: 10.1038/485585a. Nature. 2012. PMID: 22660313 No abstract available.
-
Reprogramming a broken heart.Cell Stem Cell. 2012 Jul 6;11(1):3-4. doi: 10.1016/j.stem.2012.06.014. Cell Stem Cell. 2012. PMID: 22770235
-
Induction of cardiomyocytes from cardiac fibroblasts.Circ Cardiovasc Genet. 2012 Aug 1;5(4):481-2. doi: 10.1161/CIRCGENETICS.112.964288. Circ Cardiovasc Genet. 2012. PMID: 22896018 No abstract available.
-
Reprogrammed cardiac fibroblasts to the rescue of heart failure.Circ Res. 2012 Sep 14;111(7):831-2. doi: 10.1161/CIRCRESAHA.112.279745. Circ Res. 2012. PMID: 22982872 No abstract available.
References
-
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. - PubMed
-
- Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. - PubMed
-
- Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441:1097–1099. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

