Modeling the Human Kinetic Adjustment Factor for Inhaled Volatile Organic Chemicals: Whole Population Approach versus Distinct Subpopulation Approach
- PMID: 22523487
- PMCID: PMC3317202
- DOI: 10.1155/2012/404329
Modeling the Human Kinetic Adjustment Factor for Inhaled Volatile Organic Chemicals: Whole Population Approach versus Distinct Subpopulation Approach
Abstract
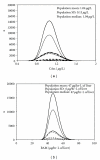
The objective of this study was to evaluate the impact of whole- and sub-population-related variabilities on the determination of the human kinetic adjustment factor (HKAF) used in risk assessment of inhaled volatile organic chemicals (VOCs). Monte Carlo simulations were applied to a steady-state algorithm to generate population distributions for blood concentrations (CAss) and rates of metabolism (RAMs) for inhalation exposures to benzene (BZ) and 1,4-dioxane (1,4-D). The simulated population consisted of various proportions of adults, elderly, children, neonates and pregnant women as per the Canadian demography. Subgroup-specific input parameters were obtained from the literature and P3M software. Under the "whole population" approach, the HKAF was computed as the ratio of the entire population's upper percentile value (99th, 95th) of dose metrics to the median value in either the entire population or the adult population. Under the "distinct subpopulation" approach, the upper percentile values in each subpopulation were considered, and the greatest resulting HKAF was retained. CAss-based HKAFs that considered the Canadian demography varied between 1.2 (BZ) and 2.8 (1,4-D). The "distinct subpopulation" CAss-based HKAF varied between 1.6 (BZ) and 8.5 (1,4-D). RAM-based HKAFs always remained below 1.6. Overall, this study evaluated for the first time the impact of underlying assumptions with respect to the interindividual variability considered (whole population or each subpopulation taken separately) when determining the HKAF.
Figures




References
-
- Dourson ML, Felter SP, Robinson D. Evolution of science-based uncertainty factors in noncancer risk assessment. Regulatory Toxicology and Pharmacology. 1996;24(2):108–120. - PubMed
-
- Dourson ML, Stara JF. Regulatory history and experimental support of uncertainty (safety) factors. Regulatory Toxicology and Pharmacology. 1983;3(3):224–238. - PubMed
-
- U.S.EPA., A review of the reference dose and reference concentration process. Risk Assessment Forum. EPA/630/P-02/00F. Washington, DC, USA, 2002.
-
- Price PS, Keenan RE, Schwab B. Defining the interindividual (intraspecies) uncertainty factor. Human and Ecological Risk Assessment. 1999;5(5):1023–1033.
-
- Dorne JLCM, Renwick AG. The refinement of uncertainty/safety factors in risk assessment by the incorporation of data on toxicokinetic variability in humans. Toxicological Sciences. 2005;86(1):20–26. - PubMed
LinkOut - more resources
Full Text Sources

