Glycobiology of immune responses
- PMID: 22524422
- PMCID: PMC3884643
- DOI: 10.1111/j.1749-6632.2012.06492.x
Glycobiology of immune responses
Abstract
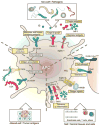
Unlike their protein "roommates" and their nucleic acid "cousins," carbohydrates remain an enigmatic arm of biology. The central reason for the difficulty in fully understanding how carbohydrate structure and biological function are tied is the nontemplate nature of their synthesis and the resulting heterogeneity. The goal of this collection of expert reviews is to highlight what is known about how carbohydrates and their binding partners-the microbial (non-self), tumor (altered-self), and host (self)-cooperate within the immune system, while also identifying areas of opportunity to those willing to take up the challenge of understanding more about how carbohydrates influence immune responses. In the end, these reviews will serve as specific examples of how carbohydrates are as integral to biology as are proteins, nucleic acids, and lipids. Here, we attempt to summarize general concepts on glycans and glycan-binding proteins (mainly C-type lectins, siglecs, and galectins) and their contributions to the biology of immune responses in physiologic and pathologic settings.
© 2012 New York Academy of Sciences.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Levine MJ, Reddy MS, Tabak LA, et al. Structural aspects of salivary glycoproteins. J Dent Res. 1987;66:436–441. - PubMed
-
- Brockhausen I, Schachter H, Stanley P. O-GalNAc Glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009. pp. 115–127. - PubMed
-
- Stanley P, Schachter H, Taniguchi N. N-Glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009. pp. 101–114. - PubMed
-
- Nothaft H, Szymanski CM. Protein glycosylation in bacteria: sweeter than ever. Nat Rev Microbiol. 2010;8:765–778. - PubMed
-
- Theodore M, Morava E. Congenital disorders of glycosylation: sweet news. Curr Opin Pediatr. 2011;23:581–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

