Design, synthesis, and antiviral activity of entry inhibitors that target the CD4-binding site of HIV-1
- PMID: 22524483
- PMCID: PMC3372086
- DOI: 10.1021/jm3002247
Design, synthesis, and antiviral activity of entry inhibitors that target the CD4-binding site of HIV-1
Abstract
The CD4 binding site on HIV-1 gp120 has been validated as a drug target to prevent HIV-1 entry to cells. Previously, we identified two small molecule inhibitors consisting of a 2,2,6,6-tetramethylpiperidine ring linked by an oxalamide to a p-halide-substituted phenyl group, which target this site, specifically, a cavity termed "Phe43 cavity". Here we use synthetic chemistry, functional assessment, and structure-based analysis to explore variants of each region of these inhibitors for improved antiviral properties. Alterations of the phenyl group and of the oxalamide linker indicated that these regions were close to optimal in the original lead compounds. Design of a series of compounds, where the tetramethylpiperidine ring was replaced with new scaffolds, led to improved antiviral activity. These new scaffolds provide insight into the surface chemistry at the entrance of the cavity and offer additional opportunities by which to optimize further these potential-next-generation therapeutics and microbicides against HIV-1.
Figures


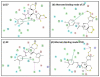
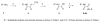

References
-
- Wyatt R, Sodroski JG. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. - PubMed
-
- Doms RW. Chemokine receptors and HIV entry. AIDS. 2001;15(Suppl 1):S34–S35. - PubMed
-
- Duffalo ML, James CW. Enfuvirtide: a novel agent for the treatment of HIV-1 infection. Ann. Pharmacother. 2003;37:1448–1456. - PubMed
-
- Hardy H, Skolnik PR. Enfuvirtide, a new fusion inhibitor for therapy of human immunodeficiency virus infection. Pharmacotherapy. 2004;24:198–211. - PubMed
-
- Meanwell NA, Kadow JF. Maraviroc, a chemokine CCR5 receptor antagonist for the treatment of HIV infection and AIDS. Curr Opin. Investig. Drugs. 2007;8:669–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

