The translational repressor T-cell intracellular antigen-1 (TIA-1) is a key modulator of Th2 and Th17 responses driving pulmonary inflammation induced by exposure to house dust mite
- PMID: 22525013
- PMCID: PMC3407291
- DOI: 10.1016/j.imlet.2012.04.001
The translational repressor T-cell intracellular antigen-1 (TIA-1) is a key modulator of Th2 and Th17 responses driving pulmonary inflammation induced by exposure to house dust mite
Abstract
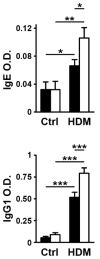
T-cell intracellular antigen-1 (TIA-1) is a translational repressor that dampens the production of proinflammatory cytokines and enzymes. In this study we investigated the role of TIA-1 in a mouse model of pulmonary inflammation induced by exposure to the allergenic extract (Df) of the house dust mite Dermatophagoides farinae. When intranasally challenged with a low dose of Df, mice lacking TIA-1 protein (Tia-1(-/-)) showed more severe airway and tissue eosinophilia, infiltration of lung bronchovascular bundles, and goblet cell metaplasia than wild-type littermates. Tia-1(-/-) mice also had higher levels of Df-specific IgE and IgG(1) in serum and ex vivo restimulated Tia-1(-/-) lymph node cells and splenocytes transcribed and released more Th2/Th17 cytokines. To evaluate the site of action of TIA-1, we studied the response to Df in bone marrow chimeras. These experiments revealed that TIA-1 acts on both hematopoietic and non-hematopoietic cells to dampen pulmonary inflammation. Our results identify TIA-1 as a negative regulator of allergen-mediated pulmonary inflammation in vivo. Thus, TIA-1 might be an important player in the pathogenesis of bronchial asthma.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Dectin-2 regulates the effector phase of house dust mite-elicited pulmonary inflammation independently from its role in sensitization.J Immunol. 2014 Feb 15;192(4):1361-71. doi: 10.4049/jimmunol.1301809. Epub 2014 Jan 22. J Immunol. 2014. PMID: 24453247 Free PMC article.
-
GPR17 regulates immune pulmonary inflammation induced by house dust mites.J Immunol. 2010 Aug 1;185(3):1846-54. doi: 10.4049/jimmunol.1001131. Epub 2010 Jun 23. J Immunol. 2010. PMID: 20574000 Free PMC article.
-
Beneficial effects of Galectin-9 on allergen-specific sublingual immunotherapy in a Dermatophagoides farinae-induced mouse model of chronic asthma.Allergol Int. 2017 Jul;66(3):432-439. doi: 10.1016/j.alit.2016.10.007. Epub 2016 Nov 19. Allergol Int. 2017. PMID: 27876361
-
Peptidoglycan recognition protein 1 enhances experimental asthma by promoting Th2 and Th17 and limiting regulatory T cell and plasmacytoid dendritic cell responses.J Immunol. 2013 Apr 1;190(7):3480-92. doi: 10.4049/jimmunol.1202675. Epub 2013 Feb 18. J Immunol. 2013. PMID: 23420883 Free PMC article.
-
House dust mite Dermatophagoides farinae augments proinflammatory mediator productions and accessory function of alveolar macrophages: implications for allergic sensitization and inflammation.J Immunol. 2003 Jan 1;170(1):528-36. doi: 10.4049/jimmunol.170.1.528. J Immunol. 2003. PMID: 12496440
Cited by
-
Noncoding RNA and its associated proteins as regulatory elements of the immune system.Nat Immunol. 2014 Jun;15(6):484-91. doi: 10.1038/ni.2887. Nat Immunol. 2014. PMID: 24840979 Review.
-
Bibliometric Overview on T-Cell Intracellular Antigens and Their Pathological Implications.Biology (Basel). 2024 Mar 19;13(3):195. doi: 10.3390/biology13030195. Biology (Basel). 2024. PMID: 38534464 Free PMC article. Review.
-
The Multifunctional Faces of T-Cell Intracellular Antigen 1 in Health and Disease.Int J Mol Sci. 2022 Jan 26;23(3):1400. doi: 10.3390/ijms23031400. Int J Mol Sci. 2022. PMID: 35163320 Free PMC article. Review.
-
The effect of oral tolerance on the allergic airway response in younger and aged mice.J Asthma. 2013 Mar;50(2):122-32. doi: 10.3109/02770903.2012.753455. Epub 2013 Jan 9. J Asthma. 2013. PMID: 23298269 Free PMC article.
-
T-cell intracellular antigens in health and disease.Cell Cycle. 2015;14(13):2033-43. doi: 10.1080/15384101.2015.1053668. Cell Cycle. 2015. PMID: 26036275 Free PMC article. Review.
References
-
- Boyce JA. Asthma 2005–2006: bench to bedside. J Allergy Clin Immunol. 2006;118(3):582–6. - PubMed
-
- Platts-Mills T, et al. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357(9258):752–6. - PubMed
-
- Platts-Mills TA, et al. Pro: The evidence for a causal role of dust mites in asthma. Am J Respir Crit Care Med. 2009;180(2):109–13. discussion 120–1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

