Critical metabolic roles of β-cell M3 muscarinic acetylcholine receptors
- PMID: 22525375
- PMCID: PMC3568704
- DOI: 10.1016/j.lfs.2012.04.010
Critical metabolic roles of β-cell M3 muscarinic acetylcholine receptors
Abstract
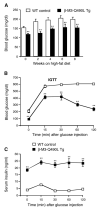
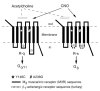
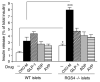
Muscarinic acetylcholine (ACh) receptors (mAChRs; M(1)-M(5)) regulate the activity of an extraordinarily large number of important physiological processes. We and others previously demonstrated that pancreatic β-cells are endowed with M(3) mAChRs which are linked to G proteins of the G(q) family. The activation of these receptors by ACh or other muscarinic agonists leads to the augmentation of glucose-induced insulin release via multiple mechanisms. Interestingly, in humans, ACh acting on human β-cell mAChRs is released from adjacent α-cells which express both choline acetyltransferase (ChAT) and the vesicular acetylcholine transporter (vAChT), indicative of the presence of a non-neuronal cholinergic system in human pancreatic islets. In order to shed light on the physiological roles of β-cell M(3) receptors, we recently generated and analyzed various mutant mouse models. Specifically, we carried out studies with mice which overexpressed M(3) receptors or mutant M(3) receptors in pancreatic β-cells or which selectively lacked M(3) receptors or M(3)-receptor-associated proteins in pancreatic β-cells. Our findings indicate that β-cell M(3) receptors play a key role in maintaining proper insulin release and whole body glucose homeostasis and that strategies aimed at enhancing signaling through β-cell M(3) receptors may prove useful to improve β-cell function for the treatment of type 2 diabetes (T2D).
Published by Elsevier Inc.
Figures
References
-
- Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–91. - PubMed
-
- Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia. 2000;43:393–410. - PubMed
-
- Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–85. - PubMed
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

