The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3
- PMID: 22525840
- PMCID: PMC4448919
- DOI: 10.1016/j.rdc.2012.03.004
The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3
Abstract
This article represents a summary of what is known of the VDR protein and its molecular mechanism of action at target genes. New methodologies now used, such as ChIP-chip and ChIP-seq, as well as novel reporter studies using large BAC clones stably transfected into culture cells or introduced as transgenes in mice, are providing new insights into how 1,25(OH)2D3-activated VDR modulates the expression of genes at single gene loci and at the level of gene networks. Many of these insights are unexpected and suggest that gene regulation is even more complex than previously appreciated. These studies also highlight new technologies and their central role in establishing fundamental biologic principles.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

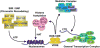
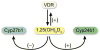

Republished from
-
The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3).Endocrinol Metab Clin North Am. 2010 Jun;39(2):255-69, table of contents. doi: 10.1016/j.ecl.2010.02.007. Endocrinol Metab Clin North Am. 2010. PMID: 20511050 Free PMC article. Review.
References
-
- Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325–49. - PubMed
-
- Sutton AL, MacDonald PN. Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol. 2003;17(5):777–91. - PubMed
-
- Zella LA, Kim S, Shevde NK, et al. Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydrox-yvitamin D3. Mol Endocrinol. 2006;20(6):1231–47. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

