Neuromyelitis optica IgG and natural killer cells produce NMO lesions in mice without myelin loss
- PMID: 22526022
- PMCID: PMC3581313
- DOI: 10.1007/s00401-012-0986-4
Neuromyelitis optica IgG and natural killer cells produce NMO lesions in mice without myelin loss
Abstract
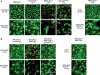
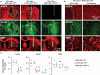
The pathogenesis of neuromyelitis optica (NMO) involves targeting of NMO-immunoglobulin G (NMO-IgG) to aquaporin-4 (AQP4) on astrocytes in the central nervous system. Prior work provided evidence for complement-dependent cytotoxicity (CDC) in NMO lesion development. Here, we show that antibody-dependent cellular cytotoxicity (ADCC), in the absence of complement, can also produce NMO-like lesions. Antibody-dependent cellular cytotoxicity was produced in vitro by incubation of mouse astrocyte cultures with human recombinant monoclonal NMO-IgG and human natural killer cells (NK-cells). Injection of NMO-IgG and NK-cells in mouse brain caused loss of AQP4 and GFAP, two characteristic features of NMO lesions, but little myelin loss. Lesions were minimal or absent following injection of: (1) control (non-NMO) IgG with NK-cells; (2) NMO-IgG and NK-cells in AQP4-deficient mice; or (3) NMO-IgG and NK-cells in wild-type mice together with an excess of mutated NMO-IgG lacking ADCC effector function. NK-cells greatly exacerbated NMO lesions produced by NMO-IgG and complement in an ex vivo spinal cord slice model of NMO, causing marked myelin loss. NMO-IgG can thus produce astrocyte injury by ADCC in a complement-independent and dependent manner, suggesting the potential involvement of ADCC in NMO pathogenesis.
Figures




References
-
- Becknell B, Caligiuri MA. Natural killer cells in innate immunity and cancer. J Immunother. 2008;31(8):685–692. - PubMed
-
- Burgoon MP, Williamson RA, Owens GP, Ghausi O, Bastidas RB, Burton DR, Gilden DH. Cloning the antibody response in humans with inflammatory CNS disease: isolation of measles virus-specific antibodies from phage display libraries of a subacute sclerosing panencephalitis brain. J Neuroimmunol. 1999;94(1–2):204–211. - PubMed
-
- Capel PJ, van de Winkel JG, van den Herik-Oudijk IE, Verbeek JS. Heterogeneity of human IgG Fc receptors. Immunomethods. 1994;4(1):25–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- DK86125/DK/NIDDK NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- RC1 DK086125/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

