Accelerated and enhanced effect of CCR5-transduced bone marrow neural stem cells on autoimmune encephalomyelitis
- PMID: 22526024
- PMCID: PMC3544339
- DOI: 10.1007/s00401-012-0989-1
Accelerated and enhanced effect of CCR5-transduced bone marrow neural stem cells on autoimmune encephalomyelitis
Abstract
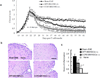
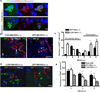
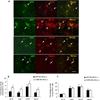
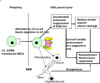
The suppressive effect of neural stem cells (NSCs) on experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), has been reported. However, the migration of NSCs to inflammatory sites was relatively slow as was the onset of rather limited clinical benefit. Lack of, or low expression of particular chemokine receptors on NSCs could be an important factor underlying the slow migration of NSCs. To enhance the therapeutic effect of NSCs, in the present study we transduced bone marrow (BM)-derived NSCs with CCR5, a receptor for CCL3, CCL4, and CCL5, chemokines that are abundantly produced in CNS-inflamed foci of MS/EAE. After i.v. injection, CCR5-NSCs rapidly reached EAE foci in larger numbers, and more effectively suppressed CNS inflammatory infiltration, myelin damage, and clinical EAE than GFP-NSCs used as controls. CCR5-NSC-treated mice also exhibited augmented remyelination and neuron/oligodendrocyte repopulation compared to PBS- or GFP-NSC-treated mice. We inferred that the critical mechanism underlying enhanced effect of CCR5-transduced NSCs on EAE is the early migration of chemokine receptor-transduced NSCs into the inflamed foci. Such migration at an earlier stage of inflammation enables NSCs to exert more effective immunomodulation, to reduce the extent of early myelin/neuron damage by creating a less hostile environment for remyelinating cells, and possibly to participate in the remyelination/neural repopulation process. These features of BM-derived transduced NSCs, combined with their easy availability (the subject's own BM) and autologous properties, may lay the groundwork for an innovative approach to rapid and highly effective MS therapy.
Figures



Similar articles
-
LINGO-1-Fc-Transduced Neural Stem Cells Are Effective Therapy for Chronic Stage Experimental Autoimmune Encephalomyelitis.Mol Neurobiol. 2017 Aug;54(6):4365-4378. doi: 10.1007/s12035-016-9994-z. Epub 2016 Jun 25. Mol Neurobiol. 2017. PMID: 27344330
-
Neurotrophin 3 transduction augments remyelinating and immunomodulatory capacity of neural stem cells.Mol Ther. 2014 Feb;22(2):440-450. doi: 10.1038/mt.2013.241. Epub 2013 Oct 17. Mol Ther. 2014. Retraction in: Mol Ther. 2023 Aug 2;31(8):2552. doi: 10.1016/j.ymthe.2023.06.014. PMID: 24247929 Free PMC article. Retracted.
-
TGFβ1 transduction enhances immunomodulatory capacity of neural stem cells in experimental autoimmune encephalomyelitis.Brain Behav Immun. 2018 Mar;69:283-295. doi: 10.1016/j.bbi.2017.11.023. Epub 2017 Dec 5. Brain Behav Immun. 2018. PMID: 29203425
-
Osthole augments therapeutic efficiency of neural stem cells-based therapy in experimental autoimmune encephalomyelitis.J Pharmacol Sci. 2014;124(1):54-65. doi: 10.1254/jphs.13144fp. J Pharmacol Sci. 2014. PMID: 24441773
-
Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies.Int J Dev Neurosci. 2013 Nov;31(7):692-700. doi: 10.1016/j.ijdevneu.2013.01.004. Epub 2013 Jan 20. Int J Dev Neurosci. 2013. PMID: 23340483 Review.
Cited by
-
The Heterogeneous Multiple Sclerosis Lesion: How Can We Assess and Modify a Degenerating Lesion?Int J Mol Sci. 2023 Jul 5;24(13):11112. doi: 10.3390/ijms241311112. Int J Mol Sci. 2023. PMID: 37446290 Free PMC article. Review.
-
Improving the neuronal differentiation efficiency of umbilical cord blood-derived mesenchymal stem cells cultivated under appropriate conditions.Iran J Basic Med Sci. 2015 Nov;18(11):1100-6. Iran J Basic Med Sci. 2015. PMID: 26949497 Free PMC article.
-
LINGO-1-Fc-Transduced Neural Stem Cells Are Effective Therapy for Chronic Stage Experimental Autoimmune Encephalomyelitis.Mol Neurobiol. 2017 Aug;54(6):4365-4378. doi: 10.1007/s12035-016-9994-z. Epub 2016 Jun 25. Mol Neurobiol. 2017. PMID: 27344330
-
GDNF Enhances Therapeutic Efficiency of Neural Stem Cells-Based Therapy in Chronic Experimental Allergic Encephalomyelitis in Rat.Stem Cells Int. 2016;2016:1431349. doi: 10.1155/2016/1431349. Epub 2016 Apr 26. Stem Cells Int. 2016. PMID: 27212951 Free PMC article.
-
Neural stem cells transplantation combined with ethyl stearate improve PD rats motor behavior by promoting NSCs migration and differentiation.CNS Neurosci Ther. 2023 Jun;29(6):1571-1584. doi: 10.1111/cns.14119. Epub 2023 Mar 16. CNS Neurosci Ther. 2023. PMID: 36924304 Free PMC article.
References
-
- Bank A, Dorazio R, Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann N Y Acad Sci. 2005;1054:308–316. - PubMed
-
- Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, Abramsky O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 2003;41:73–80. - PubMed
-
- Bonilla S, Silva A, Valdes L, Geijo E, Garcia-Verdugo JM, Martinez S. Functional neural stem cells derived from adult bone marrow. Neuroscience. 2005;133:85–95. - PubMed
-
- Calida DM, Constantinescu C, Purev E, Zhang GX, Ventura ES, Lavi E, Rostami A. Cutting edge: C3, a key component of complement activation, is not required for the development of myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis in mice. J Immunol. 2001;166:723–726. - PubMed
-
- Croxford JL, Feldmann M, Chernajovsky Y, Baker D. Different therapeutic outcomes in experimental allergic encephalomyelitis dependent upon the mode of delivery of IL-10: a comparison of the effects of protein, adenoviral or retroviral IL-10 delivery into the central nervous system. J Immunol. 2001;166:4124–4130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

