In vitro activity of MK-7655, a novel β-lactamase inhibitor, in combination with imipenem against carbapenem-resistant Gram-negative bacteria
- PMID: 22526311
- PMCID: PMC3393460
- DOI: 10.1128/AAC.05927-11
In vitro activity of MK-7655, a novel β-lactamase inhibitor, in combination with imipenem against carbapenem-resistant Gram-negative bacteria
Abstract
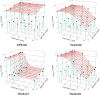
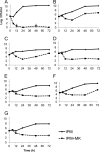
Carbapenem-resistant bacteria represent a significant treatment challenge due to the lack of active antimicrobials available. MK-7655 is a novel β-lactamase inhibitor under clinical development. We investigated the combined killing activity of imipenem and MK-7655 against four imipenem-resistant bacterial strains, using a mathematical model previously evaluated in our laboratory. Time-kill studies (TKS) were conducted with imipenem and MK-7655 against a KPC-2-producing Klebsiella pneumoniae isolate (KP6339) as well as 3 Pseudomonas aeruginosa isolates (PA24226, PA24227, and PA24228) with OprD porin deletions and overexpression of AmpC. TKS were performed using 25 clinically achievable concentration combinations in a 5-by-5 array. Bacterial burden at 24 h was determined in triplicate by quantitative culture and mathematically modeled using a three-dimensional response surface. Mathematical model assessments were evaluated experimentally using clinically relevant dosing regimens of imipenem, with or without MK-7655, in a hollow-fiber infection model (HFIM). The combination of imipenem and MK-7655 was synergistic for all strains. Interaction indices were as follows: for KP6339, 0.50 (95% confidence interval [CI], 0.42 to 0.58); for PA24226, 0.60 (95% CI, 0.58 to 0.62); for PA24227, 0.70 (95% CI, 0.66 to 0.74); and for PA24228, 0.55 (95% CI, 0.49 to 0.61). In the HFIM, imipenem plus MK-7655 considerably reduced the bacterial burden at 24 h, while failure with imipenem alone was seen against all isolates. Sustained suppression of bacterial growth at 72 h was achieved with simulated doses of 500 mg imipenem plus 500 mg MK-7655 in 2 (KP6339 and PA24227) strains, and it was achieved in an additional strain (PA24228) when the imipenem dose was increased to 1,000 mg. Additional studies are being conducted to determine the optimal dose and combinations to be used in clinical investigations.
Figures
References
-
- Castanheira M, Mendes RE, Woosley LN, Jones RN. 2011. Trends in carbapenemase-producing Escherichia coli and Klebsiella spp. from Europe and the Americas: report from the SENTRY antimicrobial surveillance programme (2007–09). J. Antimicrob. Chemother. 66:1409–1411 - PubMed
-
- Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial testing; 20th informational supplement. CLSI M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA
-
- D'Argenio DZ, Schumitzky A. 1997. ADAPT II user's guide: pharmacokinetic/pharmacodynamic systems analysis software.
-
- Davies TA, et al. 2011. Longitudinal survey of carbapenem resistance and resistance mechanisms in Enterobacteriaceae and non-fermenters from the U. S. A. in 2007–09. J. Antimicrob. Chemother. 66:2298–2307 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

