Nitrogen availability impacts oilseed rape (Brassica napus L.) plant water status and proline production efficiency under water-limited conditions
- PMID: 22526495
- PMCID: PMC3404282
- DOI: 10.1007/s00425-012-1636-8
Nitrogen availability impacts oilseed rape (Brassica napus L.) plant water status and proline production efficiency under water-limited conditions
Abstract
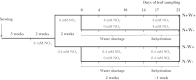
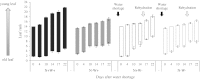
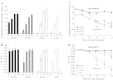
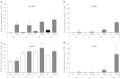
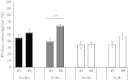
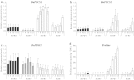
Large amounts of nitrogen (N) fertilizers are used in the production of oilseed rape. However, as low-input methods of crop management are introduced crops will need to withstand temporary N deficiency. In temperate areas, oilseed rape will also be affected by frequent drought periods. Here we evaluated the physiological and metabolic impact of nitrate limitation on the oilseed rape response to water deprivation. Different amounts of N fertilizer were applied to plants at the vegetative stage, which were then deprived of water and rehydrated. Both water and N depletion accelerated leaf senescence and reduced leaf development. N-deprived plants exhibited less pronounced symptoms of wilting during drought, probably because leaves were smaller and stomata were partially closed. Efficiency of proline production, a major stress-induced diversion of nitrogen metabolism, was assessed at different positions along the whole plant axis and related to leaf developmental stage and water status indices. Proline accumulation, preferentially in younger leaves, accounted for 25-85% of the free amino acid pool. This was mainly due to a better capacity for proline synthesis in fully N-supplied plants whether they were subjected to drought or not, as deduced from the expression patterns of the proline metabolism BnP5CS and BnPDH genes. Although less proline accumulated in the oldest leaves, a significant amount was transported from senescing to emerging leaves. Moreover, during rehydration proline was readily recycled. Our results therefore suggest that proline plays a significant role in leaf N remobilization and in N use efficiency in oilseed rape.
Figures




References
-
- Andersen MN, Heidmann T, Plauborg F. The effects of drought and nitrogen on light interception, growth and yield of winter oilseed rape. Acta Agric Scand Sect B Plant Soil Sci. 1996;46:55–67.
-
- Aufhammer W, Kübler E, Bury M. Nitrogen uptake and nitrogen residuals of winter oilseed rape and fallout rape. J Agron Crop Sci. 1994;172:255–264. doi: 10.1111/j.1439-037X.1994.tb00176.x. - DOI
-
- Barlóg P, Grzebisz W. Effect of timing and nitrogen fertilizer application on winter oilseed rape (Brassica napus L.). II. Nitrogen uptake dynamics and fertilizer efficiency. J Agron Crop Sci. 2004;190:314–323. doi: 10.1111/j.1439-037X.2004.00109.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

